当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regiodivergent and Stereoselective Synthesis of Highly Substituted 1,3-Dienes via Arylative Acyloxy Migration of Propargyl Esters
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-31 , DOI: 10.1021/jacs.3c06253 Zhimin Sun 1 , Mengfu Dai 1 , Chencheng Ding 1 , Shiqin Chen 1 , Liang-An Chen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-31 , DOI: 10.1021/jacs.3c06253 Zhimin Sun 1 , Mengfu Dai 1 , Chencheng Ding 1 , Shiqin Chen 1 , Liang-An Chen 1
Affiliation
|
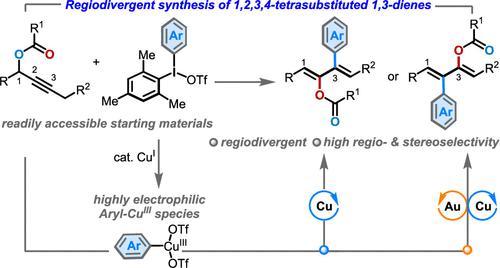
We report the first catalyst-controlled regiodivergent method that enables the synthesis of structurally diverse 1,2,3,4-tetrasubstituted conjugated dienes with excellent regio- and stereochemical outcomes from the same set of readily available propargyl esters and diaryliodonium salts. In this diene chemistry, the in situ generated, highly electrophilic aryl–CuIII complex serves not only as a π-Lewis acid catalyst for alkyne activation/acyloxy migration but also as an aryl electrophile equivalent. The competitive arylative 1,2- and 1,3-acyloxy migration patterns are exquisitely dictated by Cu and Au/Cu relay catalyses, respectively, providing a modular and attractive approach to traditionally inaccessible tetrasubstituted 1,3-dienes in a regiodivergent manner. Finally, the synthetic utility of this method is demonstrated by further synthetic derivatization of 1,3-dienes into an array of useful compounds.
中文翻译:

通过炔丙酯的芳基酰氧基迁移区域发散和立体选择性合成高度取代的 1,3-二烯
我们报告了第一种催化剂控制的区域发散方法,该方法能够从同一组容易获得的炔丙酯和二芳基碘鎓盐合成结构多样的1,2,3,4-四取代共轭二烯,并具有优异的区域和立体化学结果。在这种二烯化学中,原位生成的高亲电性芳基-Cu III络合物不仅可作为炔烃活化/酰氧基迁移的π-路易斯酸催化剂,而且还可作为芳基亲电试剂等价物。竞争性芳基化 1,2- 和 1,3-酰氧基迁移模式分别由 Cu 和 Au/Cu 中继催化精确控制,为以区域发散方式传统上难以接近的四取代 1,3-二烯提供了一种模块化且有吸引力的方法。最后,通过将 1,3-二烯进一步合成衍生为一系列有用的化合物,证明了该方法的合成效用。
更新日期:2023-07-31
中文翻译:

通过炔丙酯的芳基酰氧基迁移区域发散和立体选择性合成高度取代的 1,3-二烯
我们报告了第一种催化剂控制的区域发散方法,该方法能够从同一组容易获得的炔丙酯和二芳基碘鎓盐合成结构多样的1,2,3,4-四取代共轭二烯,并具有优异的区域和立体化学结果。在这种二烯化学中,原位生成的高亲电性芳基-Cu III络合物不仅可作为炔烃活化/酰氧基迁移的π-路易斯酸催化剂,而且还可作为芳基亲电试剂等价物。竞争性芳基化 1,2- 和 1,3-酰氧基迁移模式分别由 Cu 和 Au/Cu 中继催化精确控制,为以区域发散方式传统上难以接近的四取代 1,3-二烯提供了一种模块化且有吸引力的方法。最后,通过将 1,3-二烯进一步合成衍生为一系列有用的化合物,证明了该方法的合成效用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号