当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correction to “Viscosity and Interfacial Tension of Binary Mixtures of n-Hexadecane with Dissolved Gases Using Surface Light Scattering and Equilibrium Molecular Dynamics Simulations”
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-07-31 , DOI: 10.1021/acs.jced.3c00438 Tobias Klein , Frances D. Lenahan , Manuel Kerscher , Julius H. Jander , Michael H. Rausch , Thomas M. Koller , Andreas P. Fröba
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-07-31 , DOI: 10.1021/acs.jced.3c00438 Tobias Klein , Frances D. Lenahan , Manuel Kerscher , Julius H. Jander , Michael H. Rausch , Thomas M. Koller , Andreas P. Fröba
|
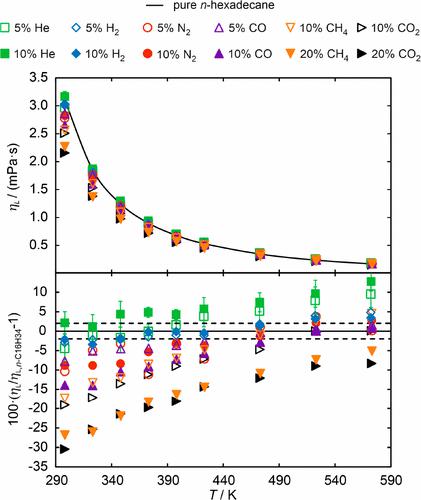
The experimental data for the dynamic viscosity ηL and the interfacial tension σ of the binary mixture consisting of n-hexadecane with dissolved hydrogen (H2) investigated at a pressure p = 3.5 MPa had to be re-evaluated due to an error in the previous data evaluation. This led to changes in both properties in the temperature range between (373.15 and 473.15) K of up to 4.0%, as indicated by the corrected data (*) in Table 2. The corrected data for the binary mixture containing H2 at p = 3.5 MPa are summarized together with all other data in Figures 1 and 2. Additionally, the deviation plot in the lower part of Figure 2 in the original publication was referring to the deviations in ηL instead of σ in the case of the two binary mixtures containing H2. Also this error was rectified in Figure 2. While the changes in ηL do not influence the discussion of the experimental data, the changes in σ require an addendum. Where the original publication stated that σ of the binary mixtures of n-hexadecane with dissolved H2 are mostly within combined uncertainties with that of pure n-hexadecane, this statement is only true for the binary mixture with xH2 ≈ 0.05. For the binary mixture with the larger amount of dissolved H2, xH2 ≈ 0.10, σ of the binary mixture is up to 9.2% smaller than that of the pure solvent at T < 423 K and agrees within combined uncertainties with the pure solvent at T ≥ 423 K.
中文翻译:

使用表面光散射和平衡分子动力学模拟修正正十六烷与溶解气体的二元混合物的粘度和界面张力
在压力p = 3.5 MPa 下研究的由正十六烷与溶解氢 (H 2 ) 组成的二元混合物的动态粘度 η L和界面张力 σ 的实验数据必须重新评估,因为之前的数据评估。这导致在 (373.15 和 473.15) K 之间的温度范围内这两种特性的变化高达 4.0%,如表 2 中的校正数据 (*) 所示。含 H 2 的二元混合物在 p 处的校正数据= 3.5 MPa 与图 1 和图 2 中的所有其他数据一起汇总。此外,原始出版物中图 2 下部的偏差图指的是 η L的偏差,而不是两个二元情况下的 σ偏差含有H 2的混合物。图 2 中也纠正了该错误。虽然 η L的变化不影响实验数据的讨论,但 σ 的变化需要附录。原始出版物指出,正十六烷与溶解的 H 2 的二元混合物的 σ大部分在与纯正十六烷的组合不确定性范围内,该陈述仅适用于具有x的二元混合物H2 ≈ 0.05。对于溶解 H 2量较多的二元混合物,x H2 ≈ 0.10,在T < 423 K时,二元混合物的 σ 比纯溶剂小 9.2%,并且在组合不确定度范围内与纯溶剂一致。T ≥ 423 K。
更新日期:2023-07-31
中文翻译:

使用表面光散射和平衡分子动力学模拟修正正十六烷与溶解气体的二元混合物的粘度和界面张力
在压力p = 3.5 MPa 下研究的由正十六烷与溶解氢 (H 2 ) 组成的二元混合物的动态粘度 η L和界面张力 σ 的实验数据必须重新评估,因为之前的数据评估。这导致在 (373.15 和 473.15) K 之间的温度范围内这两种特性的变化高达 4.0%,如表 2 中的校正数据 (*) 所示。含 H 2 的二元混合物在 p 处的校正数据= 3.5 MPa 与图 1 和图 2 中的所有其他数据一起汇总。此外,原始出版物中图 2 下部的偏差图指的是 η L的偏差,而不是两个二元情况下的 σ偏差含有H 2的混合物。图 2 中也纠正了该错误。虽然 η L的变化不影响实验数据的讨论,但 σ 的变化需要附录。原始出版物指出,正十六烷与溶解的 H 2 的二元混合物的 σ大部分在与纯正十六烷的组合不确定性范围内,该陈述仅适用于具有x的二元混合物H2 ≈ 0.05。对于溶解 H 2量较多的二元混合物,x H2 ≈ 0.10,在T < 423 K时,二元混合物的 σ 比纯溶剂小 9.2%,并且在组合不确定度范围内与纯溶剂一致。T ≥ 423 K。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号