当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid and Modular Access to Quaternary Carbons from Tertiary Alcohols via Bimolecular Homolytic Substitution
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-20 , DOI: 10.1021/jacs.3c05405
Colin A Gould 1 , Andria L Pace 1 , David W C MacMillan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-20 , DOI: 10.1021/jacs.3c05405
Colin A Gould 1 , Andria L Pace 1 , David W C MacMillan 1
Affiliation
|
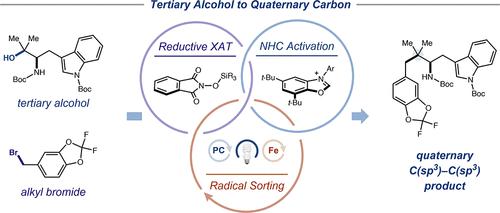
Quaternary carbons are ubiquitous in bioactive molecules; however, synthetic methods for the construction of this motif remain underdeveloped. Here, we report the synthesis of quaternary carbons from tertiary alcohols, a class of structurally diverse, bench-stable feedstocks, via the merger of photoredox catalysis and iron-mediated SH2 bond formation. This alcohol–bromide cross-coupling is enabled by a novel halogen-atom transfer (XAT) reagent, which is the first reductively activated XAT reagent to be reported. A wide variety of sterically congested quaternary products can be accessed through this mild and practical protocol including products derived from both alkylation and benzylation of tertiary fragments. We further demonstrate the synthetic utility of this method through the expedited synthesis of a liver receptor agonist and through a two-step conversion of ketones and esters to quaternary products, which enables the modular control of up to three of the four substituents on a quaternary center.
中文翻译:

通过双分子均质取代从叔醇中快速、模块化地获取季碳
季碳普遍存在于生物活性分子中。然而,构建该基序的合成方法仍然不发达。在这里,我们报道了通过光氧化还原催化和铁介导的 S H 2 键形成的结合,从叔醇(一类结构多样、实验室稳定的原料)合成季碳。这种醇-溴化物交叉偶联是通过一种新型卤素原子转移 (XAT) 试剂实现的,这是第一个被报道的还原活化 XAT 试剂。通过这种温和实用的方案可以获得多种空间拥挤的四级产物,包括源自三级片段的烷基化和苄基化的产物。我们通过肝受体激动剂的快速合成以及通过酮和酯向四元产物的两步转化进一步证明了该方法的合成效用,这使得能够对四元中心上四个取代基中的最多三个进行模块化控制。
更新日期:2023-07-20
中文翻译:

通过双分子均质取代从叔醇中快速、模块化地获取季碳
季碳普遍存在于生物活性分子中。然而,构建该基序的合成方法仍然不发达。在这里,我们报道了通过光氧化还原催化和铁介导的 S H 2 键形成的结合,从叔醇(一类结构多样、实验室稳定的原料)合成季碳。这种醇-溴化物交叉偶联是通过一种新型卤素原子转移 (XAT) 试剂实现的,这是第一个被报道的还原活化 XAT 试剂。通过这种温和实用的方案可以获得多种空间拥挤的四级产物,包括源自三级片段的烷基化和苄基化的产物。我们通过肝受体激动剂的快速合成以及通过酮和酯向四元产物的两步转化进一步证明了该方法的合成效用,这使得能够对四元中心上四个取代基中的最多三个进行模块化控制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号