当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and structure–activity studies of new rhodanine derivatives as carbonic anhydrase II, IX inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-06-30 , DOI: 10.1002/ardp.202300205 Krishna Kartheek Chinchilli 1 , Ravikumar Akunuri 1 , Shaik Mahammad Ghouse 1 , Devandla Soujanya 1 , Andrea Angeli 2 , Ramulu Parupalli 1 , Mohammed Arifuddin 3 , Venkata Madhavi Yaddanapudi 1 , Claudiu T Supuran 2 , Srinivas Nanduri 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-06-30 , DOI: 10.1002/ardp.202300205 Krishna Kartheek Chinchilli 1 , Ravikumar Akunuri 1 , Shaik Mahammad Ghouse 1 , Devandla Soujanya 1 , Andrea Angeli 2 , Ramulu Parupalli 1 , Mohammed Arifuddin 3 , Venkata Madhavi Yaddanapudi 1 , Claudiu T Supuran 2 , Srinivas Nanduri 1
Affiliation
|
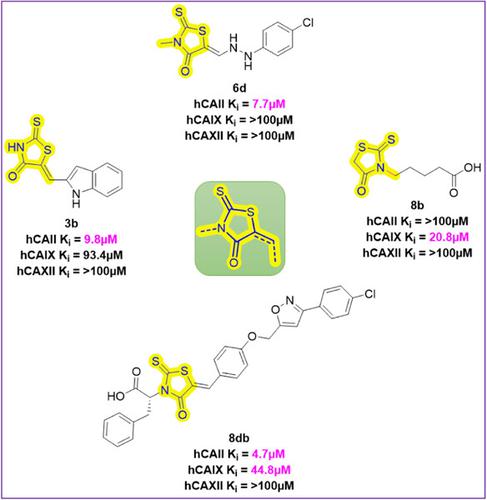
Rhodanine and its derivatives are an important class of heterocycles with diverse biological properties, including anticancer, antibacterial, and anti-mycobacterial activities. In the present work, four series of new Rhodanine derivatives were synthesized and evaluated for their inhibitory activity against carbonic anhydrase I, II, IX, and XII isoforms. Interestingly, the tested compounds exhibited good inhibitory activity against the cytosolic isoform human carbonic anhydrase (hCA) II and tumor-associated hCA IX. While the Rhodanine-benzylidene derivatives (3a–l) and Rhodanine-hydrazine derivatives (6a–e) are found to be selective against hCA II, the Rhodanine-N-carboxylate derivatives (8a–d) are found to be highly selective toward hCA IX. The Rhodanine-linked isoxazole and 1,2,4-oxadiazole derivatives (8ba, 8da, and 8db) exhibited inhibitory activity against hCA II and hCA IX. Among the tested compounds, 3b, 3j, 6d, and 8db were found to inhibit hCA II with Ki values of 9.8, 46.4, 7.7, and 4.7 µM, respectively. Furthermore, their mechanism of action is supported by molecular docking studies. Notably, the synthesized Rhodanine derivatives belong to a nonsulfonamide class of carbonic anhydrase inhibitors.
中文翻译:

作为碳酸酐酶 II、IX 抑制剂的新型绕丹宁衍生物的设计、合成和结构活性研究
绕丹宁及其衍生物是一类重要的杂环化合物,具有多种生物学特性,包括抗癌、抗菌和抗分枝杆菌活性。在目前的工作中,合成了四个系列的新绕丹宁衍生物,并评估了它们对碳酸酐酶 I、II、IX 和 XII 亚型的抑制活性。有趣的是,测试的化合物对胞质亚型人碳酸酐酶 (hCA) II 和肿瘤相关的 hCA IX 表现出良好的抑制活性。虽然绕丹宁-苯亚甲基衍生物 ( 3a–l ) 和绕丹宁-肼衍生物 ( 6a–e ) 对 hCA II 具有选择性,但绕丹宁-N-羧酸盐衍生物 ( 8a–d ) 对 hCA 具有高度选择性九.绕丹宁连接的异恶唑和 1,2,4-恶二唑衍生物(8ba、8da和8db)对 hCA II 和 hCA IX 表现出抑制活性。在测试的化合物中,发现3b、3j、6d和8db抑制 hCA II, K i值分别为 9.8、46.4、7.7 和 4.7 µM。此外,它们的作用机制得到了分子对接研究的支持。值得注意的是,合成的绕丹宁衍生物属于非磺酰胺类碳酸酐酶抑制剂。
更新日期:2023-06-30
中文翻译:

作为碳酸酐酶 II、IX 抑制剂的新型绕丹宁衍生物的设计、合成和结构活性研究
绕丹宁及其衍生物是一类重要的杂环化合物,具有多种生物学特性,包括抗癌、抗菌和抗分枝杆菌活性。在目前的工作中,合成了四个系列的新绕丹宁衍生物,并评估了它们对碳酸酐酶 I、II、IX 和 XII 亚型的抑制活性。有趣的是,测试的化合物对胞质亚型人碳酸酐酶 (hCA) II 和肿瘤相关的 hCA IX 表现出良好的抑制活性。虽然绕丹宁-苯亚甲基衍生物 ( 3a–l ) 和绕丹宁-肼衍生物 ( 6a–e ) 对 hCA II 具有选择性,但绕丹宁-N-羧酸盐衍生物 ( 8a–d ) 对 hCA 具有高度选择性九.绕丹宁连接的异恶唑和 1,2,4-恶二唑衍生物(8ba、8da和8db)对 hCA II 和 hCA IX 表现出抑制活性。在测试的化合物中,发现3b、3j、6d和8db抑制 hCA II, K i值分别为 9.8、46.4、7.7 和 4.7 µM。此外,它们的作用机制得到了分子对接研究的支持。值得注意的是,合成的绕丹宁衍生物属于非磺酰胺类碳酸酐酶抑制剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号