Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanozyme-Catalyzed Metasurface Plasmon Sensor-Based Portable Ultrasensitive Optical Quantification Platform for Cancer Biomarker Screening
Advanced Science ( IF 14.3 ) Pub Date : 2023-06-26 , DOI: 10.1002/advs.202301658 Rui Li 1 , Hongli Fan 1 , Hanlin Zhou 2 , Youqian Chen 1 , Qingcai Yu 3 , Wenjun Hu 1 , Gang L Liu 1 , Liping Huang 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2023-06-26 , DOI: 10.1002/advs.202301658 Rui Li 1 , Hongli Fan 1 , Hanlin Zhou 2 , Youqian Chen 1 , Qingcai Yu 3 , Wenjun Hu 1 , Gang L Liu 1 , Liping Huang 1, 2
Affiliation
|
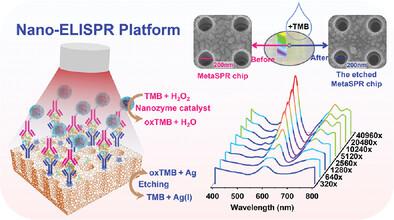
Developing plasmonic biosensors that are low-cost, portable, and relatively simple to operate remains challenging. Herein, a novel metasurface plasmon-etch immunosensor is described, namely a nanozyme-linked immunosorbent surface plasmon resonance biosensor, for the ultrasensitive and specific detection of cancer biomarkers. Gold-silver composite nano cup array metasurface plasmon resonance chip and artificial nanozyme-labeled antibody are used in two-way sandwich analyte detection. Changes in the biosensor's absorption spectrum are measured before and after chip surface etching, which can be applied to immunoassays without requiring separation or amplification. The device achieved a limit of alpha-fetoprotein (AFP) detection < 21.74 fM, three orders of magnitude lower than that of commercial enzyme-linked immunosorbent assay kits. Additionally, carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA125) are used for quantitative detection to verify the universality of the platform. More importantly, the accuracy of the platform is verified using 60 clinical samples; compared with the hospital results, the three biomarkers achieve high sensitivity (CEA: 95.7%; CA125: 90.9%; AFP: 86.7%) and specificity (CEA: 97.3%; CA125: 93.9%; AFP: 97.8%). Due to its rapidity, ease of use, and high throughput, the platform has the potential for high-throughput rapid detection to facilitate cancer screening or early diagnostic testing in biosensing.
中文翻译:

基于纳米酶催化超表面等离子体传感器的便携式超灵敏光学定量平台,用于癌症生物标志物筛查
开发低成本、便携式且操作相对简单的等离子体生物传感器仍然具有挑战性。本文描述了一种新型超表面等离子体蚀刻免疫传感器,即纳米酶联免疫吸附表面等离子体共振生物传感器,用于癌症生物标志物的超灵敏和特异性检测。金银复合纳米杯阵列超表面等离子体共振芯片和人工纳米酶标记抗体用于双向夹心分析物检测。测量芯片表面蚀刻前后生物传感器吸收光谱的变化,可应用于免疫测定,无需分离或放大。该装置实现了甲胎蛋白 (AFP) 检测限 < 21.74 fM,比商用酶联免疫吸附检测试剂盒低三个数量级。此外,还利用癌胚抗原(CEA)和糖抗原125(CA125)进行定量检测,以验证该平台的通用性。更重要的是,该平台的准确性是使用60个临床样本进行验证的;与医院结果相比,三种生物标志物均具有较高的敏感性(CEA:95.7%;CA125:90.9%;AFP:86.7%)和特异性(CEA:97.3%;CA125:93.9%;AFP:97.8%)。由于其快速、易于使用和高通量,该平台具有高通量快速检测的潜力,以促进生物传感中的癌症筛查或早期诊断测试。
更新日期:2023-06-26
中文翻译:

基于纳米酶催化超表面等离子体传感器的便携式超灵敏光学定量平台,用于癌症生物标志物筛查
开发低成本、便携式且操作相对简单的等离子体生物传感器仍然具有挑战性。本文描述了一种新型超表面等离子体蚀刻免疫传感器,即纳米酶联免疫吸附表面等离子体共振生物传感器,用于癌症生物标志物的超灵敏和特异性检测。金银复合纳米杯阵列超表面等离子体共振芯片和人工纳米酶标记抗体用于双向夹心分析物检测。测量芯片表面蚀刻前后生物传感器吸收光谱的变化,可应用于免疫测定,无需分离或放大。该装置实现了甲胎蛋白 (AFP) 检测限 < 21.74 fM,比商用酶联免疫吸附检测试剂盒低三个数量级。此外,还利用癌胚抗原(CEA)和糖抗原125(CA125)进行定量检测,以验证该平台的通用性。更重要的是,该平台的准确性是使用60个临床样本进行验证的;与医院结果相比,三种生物标志物均具有较高的敏感性(CEA:95.7%;CA125:90.9%;AFP:86.7%)和特异性(CEA:97.3%;CA125:93.9%;AFP:97.8%)。由于其快速、易于使用和高通量,该平台具有高通量快速检测的潜力,以促进生物传感中的癌症筛查或早期诊断测试。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号