当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Synthesis of Functionalized 4-Substituted-4H-Benzo[d][1,3]oxazines via Intramolecular Cyclization of ortho-Amide-N-tosylhydrazones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-16 , DOI: 10.1021/acs.joc.3c00534 Jun Yan 1 , Christine Tran 1 , Pascal Retailleau 2 , Mouad Alami 1 , Abdallah Hamze 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-06-16 , DOI: 10.1021/acs.joc.3c00534 Jun Yan 1 , Christine Tran 1 , Pascal Retailleau 2 , Mouad Alami 1 , Abdallah Hamze 1
Affiliation
|
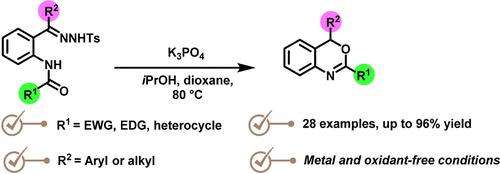
Functionalized 4-aryl-4H-benzo[d][1,3]oxazines are synthesized under transition-metal-free conditions using ortho-amide-N-tosylhydrazones. This synthetic method uses readily available N-tosylhydrazones as the diazo compound precursors and involves an intramolecular ring closure reaction mediated by a protic polar additive (iPrOH). A wide range of functionalized oxazines are obtained by this straightforward method in good to excellent yields. Furthermore, the viability of our strategy is illustrated by the gram-scale elaboration of a bromo-substituted 4H-benzo[d][1,3]oxazine and its post-functionalization by palladium-catalyzed cross-couplings.
中文翻译:

通过邻酰胺-N-甲苯磺酰腙分子内环化无催化剂合成功能化4-取代-4H-苯并[d][1,3]恶嗪
使用邻酰胺-N-甲苯磺酰腙在无过渡金属的条件下合成官能化的4-芳基-4H-苯并[ d ][1,3]恶嗪。该合成方法使用容易获得的N-甲苯磺酰腙作为重氮化合物前体,并涉及由质子极性添加剂( i PrOH)介导的分子内闭环反应。通过这种简单的方法可以以良好到优异的收率获得各种官能化恶嗪。此外,我们的策略的可行性通过溴代 4 H-苯并[ d][1,3]恶嗪及其通过钯催化交叉偶联的后官能化。
更新日期:2023-06-16
中文翻译:

通过邻酰胺-N-甲苯磺酰腙分子内环化无催化剂合成功能化4-取代-4H-苯并[d][1,3]恶嗪
使用邻酰胺-N-甲苯磺酰腙在无过渡金属的条件下合成官能化的4-芳基-4H-苯并[ d ][1,3]恶嗪。该合成方法使用容易获得的N-甲苯磺酰腙作为重氮化合物前体,并涉及由质子极性添加剂( i PrOH)介导的分子内闭环反应。通过这种简单的方法可以以良好到优异的收率获得各种官能化恶嗪。此外,我们的策略的可行性通过溴代 4 H-苯并[ d][1,3]恶嗪及其通过钯催化交叉偶联的后官能化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号