当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective and Enantioconvergent Iron-Catalyzed C(sp3)-H Aminations to Chiral 2-Imidazolidinones
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-04-24 , DOI: 10.1002/cjoc.202300162 Tianjiao Cui 1 , Chen‐Xi Ye 1 , Jordan Thelemann 1 , Daniel Jenisch 1 , Eric Meggers 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-04-24 , DOI: 10.1002/cjoc.202300162 Tianjiao Cui 1 , Chen‐Xi Ye 1 , Jordan Thelemann 1 , Daniel Jenisch 1 , Eric Meggers 1
Affiliation
|
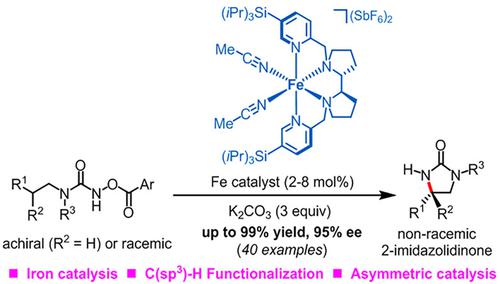
Enantioselective or enantioconvergent iron-catalyzed ring-closing C(sp3)-H aminations of N-aroyloxyurea through intermediate iron nitrene species provide chiral 2-imidazolidinones in up to 99% yield and with up to 95% ee (40 examples). This is a rare example in which sustainable iron catalysis is combined with C(sp3)-H amination and asymmetric catalysis. Chiral 2-imidazolidinones are prevalent structural motifs in bioactive molecules and can also be hydrolyzed to valuable chiral vicinal diamines in a single step.
中文翻译:

对映选择性和对映收敛铁催化 C(sp3)-H 胺化为手性 2-咪唑啉酮
N-芳酰氧基脲通过中间铁氮烯物种进行对映选择性或对映会聚的铁催化闭环 C(sp 3 )-H 胺化,提供手性 2-咪唑啉酮,产率高达 99%,ee 高达 95%(40 个实例)。这是可持续铁催化与C(sp 3 )-H胺化和不对称催化相结合的罕见例子。手性 2-咪唑啉酮是生物活性分子中常见的结构基序,也可以一步水解为有价值的手性邻二胺。
更新日期:2023-04-24

中文翻译:

对映选择性和对映收敛铁催化 C(sp3)-H 胺化为手性 2-咪唑啉酮
N-芳酰氧基脲通过中间铁氮烯物种进行对映选择性或对映会聚的铁催化闭环 C(sp 3 )-H 胺化,提供手性 2-咪唑啉酮,产率高达 99%,ee 高达 95%(40 个实例)。这是可持续铁催化与C(sp 3 )-H胺化和不对称催化相结合的罕见例子。手性 2-咪唑啉酮是生物活性分子中常见的结构基序,也可以一步水解为有价值的手性邻二胺。






























 京公网安备 11010802027423号
京公网安备 11010802027423号