当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
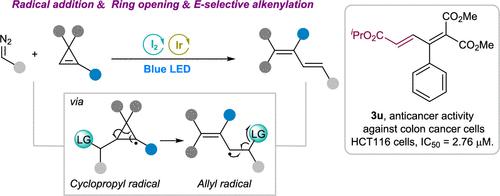
Carbon-Centered Radical with Leaving Group-Mediated Ring Opening of Cyclopropenes via the Rearrangement of Cyclopropyl to the Allyl Radical: A General Access to Multisubstituted 1,3-Dienes
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-04 , DOI: 10.1021/acscatal.3c00619 Geng-Xin Liu 1 , Xiao-Ting Jie 1 , Xing-lin Li 1 , Li-Sheng Yang 1 , Huang Qiu 1 , Wen-Hao Hu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-04 , DOI: 10.1021/acscatal.3c00619 Geng-Xin Liu 1 , Xiao-Ting Jie 1 , Xing-lin Li 1 , Li-Sheng Yang 1 , Huang Qiu 1 , Wen-Hao Hu 1
Affiliation
|
We design and develop a general strategy for the assembly of multisubstituted 1,3-diene derivatives. This methodology proceeds through the cleavage of cyclopropenes via the rearrangement of the cyclopropyl radical to the allyl radical after the addition of a carbon-centered radical with a leaving group onto the strained double bond, leading to 1,3-diene products with the release of the leaving group. This approach represents a reaction mode for carbon-centered radical-mediated functionalization of cyclopropenes with ring cleavage. The transformation occurs under mild reaction conditions and shows high functional group tolerance. These highly valuable and modifiable 1,3-diene products show good antitumor activity against HCT116 cells.
中文翻译:

以碳为中心的自由基与离去基团介导的环丙烯开环通过环丙基重排为烯丙基自由基:多取代 1,3-二烯的一般途径
我们设计并开发了组装多取代 1,3-二烯衍生物的总体策略。该方法在将带有离去基团的以碳为中心的自由基添加到紧张的双键上之后,通过环丙基重排为烯丙基来裂解环丙烯,从而导致 1,3-二烯产物释放离去基团。这种方法代表了一种以碳为中心的自由基介导的环丙烯裂解功能化反应模式。转化发生在温和的反应条件下,并显示出高官能团耐受性。这些极具价值且可修饰的 1,3-二烯产品对 HCT116 细胞显示出良好的抗肿瘤活性。
更新日期:2023-04-04
中文翻译:

以碳为中心的自由基与离去基团介导的环丙烯开环通过环丙基重排为烯丙基自由基:多取代 1,3-二烯的一般途径
我们设计并开发了组装多取代 1,3-二烯衍生物的总体策略。该方法在将带有离去基团的以碳为中心的自由基添加到紧张的双键上之后,通过环丙基重排为烯丙基来裂解环丙烯,从而导致 1,3-二烯产物释放离去基团。这种方法代表了一种以碳为中心的自由基介导的环丙烯裂解功能化反应模式。转化发生在温和的反应条件下,并显示出高官能团耐受性。这些极具价值且可修饰的 1,3-二烯产品对 HCT116 细胞显示出良好的抗肿瘤活性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号