Electrochimica Acta ( IF 5.5 ) Pub Date : 2023-04-01 , DOI: 10.1016/j.electacta.2023.142351 Mohammad Numair Ansari 1 , Stephanie Sarrouf 2 , Muhammad Fahad Ehsan 2 , Sumaira Manzoor 1 , Muhammad Naeem Ashiq 1 , Akram N Alshawabkeh 2
|
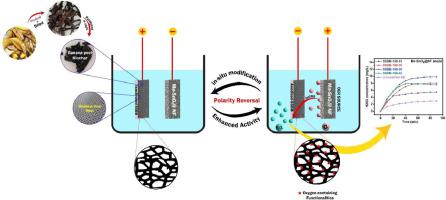
The fabrication of a cost-efficient cathode is critical for in-situ electrochemical generation of hydrogen peroxide (H2O2) to remove persistent organic pollutants from groundwater. Herein, we tested a stainless-steel (SS) mesh wrapped banana-peel derived biochar (BB) cathode for in-situ H2O2 electrogeneration to degrade bromophenol blue (BPB) and Congo red (CR) dyes. Furthermore, polarity reversal is evaluated for the activation of BB surface via introduction of various oxygen containing functionalities that serve as active sites for the oxygen reduction reaction (ORR) to generate H2O2. Various parameters including the BB mass, current, as well as the solution pH have been optimized to evaluate the cathode performance for efficient H2O2 generation. The results reveal formation of up to 9.4 mg/L H2O2 using 2.0 g BB and 100 mA current in neutral pH with no external oxygen supply with a manganese doped tin oxide deposited nickel foam (Mn-SnO2@NF) anode to facilitate the oxygen evolution reaction (OER). This iron-free electrofenton (EF) like process enabled by the SSBB cathode facilitates efficient degradation of BPB and CR dyes with 87.44 and 83.63% removal efficiency, respectively after 60 min. A prolonged stability test over 10 cycles demonstrates the effectiveness of polarity reversal toward continued removal efficiency as an added advantage. Moreover, Mn-SnO2@NF anode used for the OER was also replaced with stainless steel (SS) mesh anode to investigate the effect of oxygen evolution on H2O2 generation. Although Mn-SnO2@NF anode exhibits better oxygen evolution potential with reduced Tafel slope, SS mesh anode is discussed to be more cost-efficient for further studies.
中文翻译:

极性反转增强香蕉皮生物炭阴极原位电化学合成 H2O2 用于水修复
制造具有成本效益的阴极对于原位电化学产生过氧化氢(H 2 O 2 )以去除地下水中的持久性有机污染物至关重要。在此,我们测试了不锈钢(SS)网包裹的香蕉皮生物炭(BB)阴极,用于原位H 2 O 2电发电,以降解溴酚蓝(BPB)和刚果红(CR)染料。此外,通过引入各种含氧官能团来评估BB表面活化的极性反转,这些含氧官能团充当氧还原反应(ORR)产生H 2 O 2 的活性位点。包括 BB 质量、电流以及溶液 pH 值在内的各种参数均经过优化,以评估有效生成 H 2 O 2的阴极性能。结果表明,在中性 pH 值下,使用 2.0 g BB 和 100 mA 电流,无需外部供氧,使用锰掺杂氧化锡沉积镍泡沫 (Mn-SnO 2 @NF) 阳极可形成高达 9.4 mg/LH 2 O 2 ,以促进析氧反应(OER)。这种由 SSBB 阴极实现的无铁电芬顿 (EF) 类似过程促进了 BPB 和 CR 染料的高效降解,60 分钟后去除效率分别为 87.44% 和 83.63%。超过 10 个循环的长时间稳定性测试证明了极性反转对于持续去除效率的有效性,这是一个额外的优势。 此外,用于OER的Mn-SnO 2 @NF阳极也被替换为不锈钢(SS)网阳极,以研究析氧对H 2 O 2生成的影响。尽管Mn-SnO 2 @NF阳极表现出更好的析氧潜力并降低了Tafel斜率,但SS网状阳极被认为更具成本效益,有利于进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号