Fuel Processing Technology ( IF 7.2 ) Pub Date : 2023-03-04 , DOI: 10.1016/j.fuproc.2023.107722 Yu Tang , Yi Cui , Gaosheng Ren , Ke Ma , Xiaoxun Ma , Chengyi Dai , Chunshan Song
|
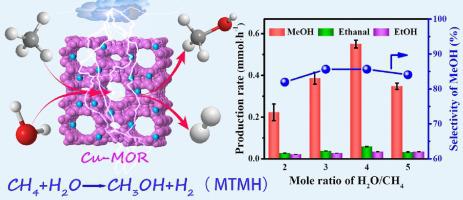
In this study, the copper-mordenite zeolite (Cu-MOR) and non-thermal plasma were used to produce methanol and hydrogen from methane and water at low temperature (120 °C) without CO2 formation. Energy efficiency for methanol and hydrogen were 68.8 mmol·kJ−1 and 562.3 mmol·kJ−1, respectively, and the selectivity of methanol in liquid products reached 86%. The reaction mechanism likely involves methane being excited to methyl (·CH3) and hydrogen radicals (·H) under DBD plasma conditions, which can then adsorb on the catalyst surface at the Cu2-(μ-O)2+ active sites, accompanied by water co-adsorption to facilitate formation and desorption of methanol. XPS analysis showed that with the prolongation of reaction time, Cu2-(μ-O)2+ was reduced to Cu+ and Cu(II) hydroxide, and the catalyst had carbon deposition, resulting in the decrease of catalytic activity. It is also worth noting that in an oxygen atmosphere, Cu2-(μ-O)2+ regenerates rapidly and eliminates catalyst carbon deposits. Trace oxygen was introduced into the reactor, and the reaction was carried out for 30 h, and the catalytic activity did not decrease.
中文翻译:

使用非热等离子体和 Cu-丝光沸石催化剂从甲烷和水中一步合成甲醇和氢气
在这项研究中,铜丝光沸石 (Cu-MOR) 和非热等离子体用于在低温 (120 °C) 下从甲烷和水中生产甲醇和氢气,而不会形成 CO 2。甲醇和氢气的能量效率分别为68.8 mmol·kJ -1和562.3 mmol·kJ -1,液体产物中甲醇的选择性达到86%。反应机制可能涉及甲烷在 DBD 等离子体条件下被激发为甲基 (·CH 3 ) 和氢自由基 (·H),然后吸附在催化剂表面的 Cu 2 -(μ-O) 2+活性位点,伴随着水的共吸附,以促进甲醇的形成和解吸。XPS分析表明,随着反应时间的延长,Cu 2 -(μ-O) 2+被还原为Cu +和Cu(II)氢氧化物,催化剂出现积炭,导致催化活性降低。还值得注意的是,在氧气气氛中,Cu 2 -(μ-O) 2+会迅速再生并消除催化剂积碳。向反应器中通入微量氧气,反应30 h,催化活性没有下降。





























 京公网安备 11010802027423号
京公网安备 11010802027423号