Results in Chemistry ( IF 2.5 ) Pub Date : 2023-01-18 , DOI: 10.1016/j.rechem.2023.100785 Jussara Lopes de Miranda , Bernardo Lages Rodrigues , Luiza Cristina de Moura , Guilherme Sales da Rocha , Suzane de Sant'Ana Oliveira
|
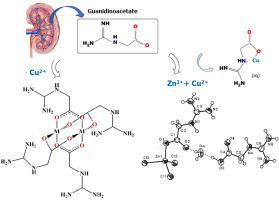
Guanidinoacetic acid (GAA) is an amino acid involved in several biological processes including renal activity since it is synthesized mainly in the kidneys, insulin metabolism, creatine/phosphocreatine synthesis and recently as experimental nutrient for multiple sclerosis treatment. Given its biological role, some interactions between GAA and its complexation with essential and toxic metal ions have been previously investigated, both in aqueous medium with Ni(II), Co(II), Cu(II), Zn(II), Cd(II), and Pb(II), as well as in the synthesis of complexes in solid phase. This study presents the versatile coordination modes of GAA and reports its novel solid zinc complex. GAA complexation ranges from the usual α-nitrogen and oxygen bidentate coordination to a paddlewheel monodentate-oxygen mode in a Cu2GAA4 complex. The novel solid GAA zinc complex has only been succeeded to be synthesized in the presence of copper(II) salt, being characterized by single-crystal structure refinement using X-ray diffraction and mid and far-Fourier Transform Infrared spectroscopy. The results showed that the synthesized complex, (HGAA)[Zn(GAA)Cl3].H2O (1) , exhibits a tetrahedral geometry with an oxygen-carboxylate GAA monodentate coordination complemented by three chloride ligands and a water bridge linked to a monoprotonated GAA species. Hydrogen bonds play a crucial role in the stabilization of 1, especially between Zn(GAA)Cl3- and its cation ion, HGAA+. Infrared spectroscopy of 1 presented a band at 412 cm-1 and three bands at 291, 273 and 239 cm-1, corresponding to Zn-O and Zn-Cl stretching modes, respectively. The different coordination modes of guanidinoacetate ion and its complexation with zinc and copper(II), as well as the way the hydrogen bonds stabilize their structures may help to further understand the diverse biochemical processes involved in this amino acid.
中文翻译:

胍基乙酸配位模式的多样性及其新型锌络合物的合成
胍基乙酸 (GAA) 是一种氨基酸,参与多种生物过程,包括肾脏活动,因为它主要在肾脏中合成、胰岛素代谢、肌酸/磷酸肌酸合成,最近作为多发性硬化症治疗的实验营养物。鉴于其生物学作用,GAA 与其与必需和有毒金属离子的络合之间的一些相互作用之前已经进行了研究,无论是在含有 Ni(II)、Co(II)、Cu(II)、Zn(II)、Cd( II), 和 Pb(II), 以及固相配合物的合成。本研究介绍了 GAA 的多种配位模式,并报道了其新型固体锌络合物。GAA 络合的范围从通常的 α-氮和氧双齿配位到 Cu 2 GAA中的桨轮单齿-氧模式4复杂。新型固体 GAA 锌络合物仅在铜 (II) 盐存在下成功合成,其特征在于使用 X 射线衍射和中远傅里叶变换红外光谱进行单晶结构细化。结果表明合成的络合物(HGAA)[Zn(GAA)Cl 3 ] 。H 2 O ( 1 ) 具有四面体几何形状,具有氧-羧酸盐 GAA 单齿配位,辅以三个氯配体和一个与 单质子化 GAA 物种相连的水桥。氢键在1的稳定中起着至关重要的作用,尤其是在 Zn(GAA)Cl 3-及其阳离子 HGAA +之间 . 1的红外光谱在412 cm -1 处出现一个波段,在291、273和239 cm -1 处出现三个波段,分别对应于Zn-O和Zn-Cl伸缩模式。胍基乙酸根离子的不同配位模式及其与锌和铜 (II) 的络合,以及氢键稳定其结构的方式可能有助于进一步了解该氨基酸所涉及的多种生化过程。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号