当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox-Catalyzed Synthesis of β-Amino Alcohols: Hydroxymethylation of Imines with α-Silyl Ether as Hydroxymethyl Radical Precursor
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-23 , DOI: 10.1021/acs.orglett.2c03633 Arjun Gontala 1 , Hyunho Huh 1 , Sang Kook Woo 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-23 , DOI: 10.1021/acs.orglett.2c03633 Arjun Gontala 1 , Hyunho Huh 1 , Sang Kook Woo 1
Affiliation
|
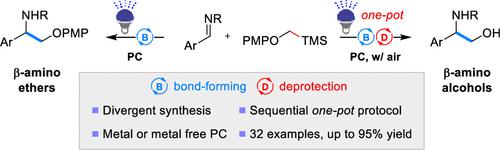
Carbon–carbon bond formation is an efficient approach for the synthesis of amino alcohols using two simple starting materials. Herein, we present a novel method for a divergent synthesis of β-amino ethers and β-amino alcohols in a sequential one-pot protocol under high-efficiency, mild, and metal- or metal-free conditions. Especially, TMSCH2OPMP was developed as a synthetic equivalent of α-hydroxymethyl radical in an in situ photocatalyzed oxidative PMP group deprotection strategy under air. A preliminary mechanistic investigation provides evidence for reaction mechanism involving a photoinduced α-alkoxy methyl radical and superoxide.
中文翻译:

光氧化还原催化合成 β-氨基醇:以 α-甲硅烷基醚为羟甲基自由基前体的亚胺羟甲基化
碳-碳键形成是使用两种简单的起始原料合成氨基醇的有效方法。在此,我们提出了一种新方法,用于在高效、温和和无金属或无金属条件下,通过连续的一锅法合成 β-氨基醚和 β-氨基醇。特别是,TMSCH 2 OPMP 被开发为在空气下原位光催化氧化 PMP 基团脱保护策略中的 α-羟甲基自由基的合成等价物。初步的机理研究为涉及光诱导的 α-烷氧基甲基自由基和超氧化物的反应机理提供了证据。
更新日期:2022-12-23
中文翻译:

光氧化还原催化合成 β-氨基醇:以 α-甲硅烷基醚为羟甲基自由基前体的亚胺羟甲基化
碳-碳键形成是使用两种简单的起始原料合成氨基醇的有效方法。在此,我们提出了一种新方法,用于在高效、温和和无金属或无金属条件下,通过连续的一锅法合成 β-氨基醚和 β-氨基醇。特别是,TMSCH 2 OPMP 被开发为在空气下原位光催化氧化 PMP 基团脱保护策略中的 α-羟甲基自由基的合成等价物。初步的机理研究为涉及光诱导的 α-烷氧基甲基自由基和超氧化物的反应机理提供了证据。






























 京公网安备 11010802027423号
京公网安备 11010802027423号