当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of highly potent synthetic opioid nitazene analogs and their positional isomers
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2022-11-27 , DOI: 10.1002/dta.3415 Tatsuyuki Kanamori 1 , Yuki Okada 1 , Hiroki Segawa 1 , Tadashi Yamamuro 1 , Kenji Kuwayama 1 , Kenji Tsujikawa 1 , Yuko T Iwata 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2022-11-27 , DOI: 10.1002/dta.3415 Tatsuyuki Kanamori 1 , Yuki Okada 1 , Hiroki Segawa 1 , Tadashi Yamamuro 1 , Kenji Kuwayama 1 , Kenji Tsujikawa 1 , Yuko T Iwata 1
Affiliation
|
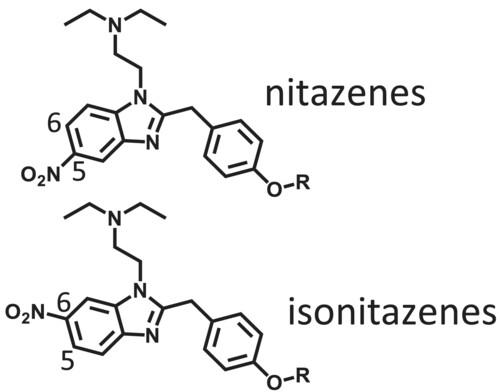
Four nitazenes (metonitazene, etonitazene, protonitazene, and isotonitazene), highly potent benzimidazole synthetic opioids, and their four nitro group positional isomers (isonitazenes) were synthesized and analyzed using infrared (IR) spectroscopy, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS). In addition, the agonistic activity of all compounds at the human μ-opioid receptor was measured using a cell-based assay system. In the IR spectra, characteristic peaks for nitazenes and isonitazenes were observed. In GC/MS, all compounds were well separated on the chromatogram, although distinguishing nitazenes from the corresponding isonitazenes by electron ionization mass spectra was difficult. In LC/MS, all compounds were detected in both positive and negative modes of electrospray ionization. Characteristic fragment ions were observed in the product ion spectra of isonitazenes, enabling nitazenes to be distinguished from isonitazenes. All nitazenes tested demonstrated higher agonistic activity at the human μ-opioid receptors than the synthetic opioid fentanyl. The agonistic activities of isonitazenes were 11–35 times lower than those of the corresponding nitazenes. However, iso-etonitazene and iso-isotonitazene showed moderate activity similar to that of fentanyl, indicating that these drugs could cause poisoning at a comparable level as fentanyl, if these drugs are abused in the future.
中文翻译:

高效合成阿片类药物硝氮苯类似物及其位置异构体的分析
使用红外 (IR) 光谱、气相色谱/质谱 (GC/MS) 合成并分析了四种硝硝氮(metonitazene、etonitazene、protonitazene 和 isotonitazene)、高效苯并咪唑合成阿片类药物及其四种硝基位置异构体(异硝氮)和液相色谱/质谱 (LC/MS)。此外,使用基于细胞的测定系统测量了所有化合物对人类 μ-阿片受体的激动活性。在 IR 光谱中,观察到烟硝氮和异硝氮的特征峰。在 GC/MS 中,所有化合物在色谱图上都得到了很好的分离,尽管通过电子电离质谱很难区分硝氮腈和相应的异硝氮。在 LC/MS 中,所有化合物均在正电喷雾电离和负电喷雾电离模式下检测到。在异硝氮酮的产物离子谱中观察到特征碎片离子,从而能够将硝硝氮酮与异硝氮酮区分开来。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。
更新日期:2022-11-27
中文翻译:

高效合成阿片类药物硝氮苯类似物及其位置异构体的分析
使用红外 (IR) 光谱、气相色谱/质谱 (GC/MS) 合成并分析了四种硝硝氮(metonitazene、etonitazene、protonitazene 和 isotonitazene)、高效苯并咪唑合成阿片类药物及其四种硝基位置异构体(异硝氮)和液相色谱/质谱 (LC/MS)。此外,使用基于细胞的测定系统测量了所有化合物对人类 μ-阿片受体的激动活性。在 IR 光谱中,观察到烟硝氮和异硝氮的特征峰。在 GC/MS 中,所有化合物在色谱图上都得到了很好的分离,尽管通过电子电离质谱很难区分硝氮腈和相应的异硝氮。在 LC/MS 中,所有化合物均在正电喷雾电离和负电喷雾电离模式下检测到。在异硝氮酮的产物离子谱中观察到特征碎片离子,从而能够将硝硝氮酮与异硝氮酮区分开来。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。所测试的所有硝氮酮对人类 μ-阿片受体的激动活性均高于合成阿片芬太尼。异硝西林的激动活性比相应的硝西西林低 11-35 倍。然而,iso-etonitazene 和 iso-isotonitazene 显示出与芬太尼相似的中等活性,表明如果将来滥用这些药物,这些药物可能会导致与芬太尼相当的中毒水平。





























 京公网安备 11010802027423号
京公网安备 11010802027423号