Biochimie ( IF 3.3 ) Pub Date : 2022-11-26 , DOI: 10.1016/j.biochi.2022.11.013 Eva Judy 1 , Nand Kishore 1
|
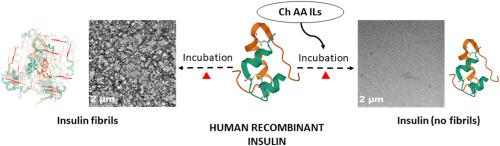
We have synthesized biocompatible ionic liquids (ILs) with choline as cation and amino acids as anions to explore their potential towards prevention of fibrillation in insulin and the obtain corresponding mechanistic insights. This has been achieved by examining the effect of these ILs on insulin at the nucleation, elongation and maturation stages of the fibrillation process. A combination of high sensitivity isothermal titration calorimetry (ITC) and differential scanning calorimetry (DSC) have been employed along with spectroscopy and microscopy to evaluate interaction of the ILs at each stage of fibrillation quantitatively. Choline glycinate is observed to provide maximum stabilization to insulin compared to that provided by choline prolinate, choline leucinate, and choline valinate. This increased thermal stabilization has direct correlation with the extent of reduction in the fibrillation of insulin by ILs determined using Thioflavin T and 8-anilinonaphthalene sulfonate based fluorescence assays. ITC has permitted understanding nature of interaction of the ILs with the protein at different fibrillation stages in terms of standard molar enthalpy of interaction whereas DSC has enabled understanding the extent of reduction in thermal stability of the protein at these stages. These ILs are able to completely inhibit formation of insulin aggregates at a concentration of 50 mM. Stabilization of proteins by ILs could be explained based on involvement of preferential hydration process. The work provides biocompatible IL based approach in achieving stability and prevention of fibrillation in insulin.
中文翻译:

基于生物相容性胆碱氨基酸的离子液体预防胰岛素纤颤:生物物理学见解
我们合成了以胆碱为阳离子、氨基酸为阴离子的生物相容性离子液体 (IL),以探索它们预防胰岛素纤颤的潜力,并获得相应的机理见解。这是通过检查这些 IL 在原纤化过程的成核、伸长和成熟阶段对胰岛素的影响来实现的。高灵敏度等温滴定量热法 (ITC) 和差示扫描量热法 (DSC) 的组合已与光谱学和显微镜一起使用,以定量评估原纤维化每个阶段 IL 的相互作用。与脯氨酸胆碱、亮氨酸胆碱和缬氨酸胆碱提供的稳定性相比,甘氨酸胆碱被观察到为胰岛素提供最大稳定性。这种增加的热稳定性与使用基于硫黄素 T 和 8-苯胺萘磺酸盐的荧光测定法确定的 IL 减少胰岛素原纤维化的程度直接相关。ITC 允许根据标准摩尔相互作用焓了解 IL 与蛋白质在不同原纤化阶段相互作用的性质,而 DSC 能够了解蛋白质在这些阶段热稳定性降低的程度。这些 IL 在 50 mM 的浓度下能够完全抑制胰岛素聚集体的形成。ILs 对蛋白质的稳定作用可以基于优先水合过程的参与来解释。这项工作提供了基于生物相容性 IL 的方法来实现胰岛素的稳定性和预防原纤维化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号