当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Mediated Radical Silyl-Oximation of Activated Alkenes Using tert-Butyl Nitrite and Silanes
Organic Letters ( IF 4.9 ) Pub Date : 2022-11-10 , DOI: 10.1021/acs.orglett.2c03644 Stefanie Plöger 1 , Armido Studer 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-11-10 , DOI: 10.1021/acs.orglett.2c03644 Stefanie Plöger 1 , Armido Studer 1
Affiliation
|
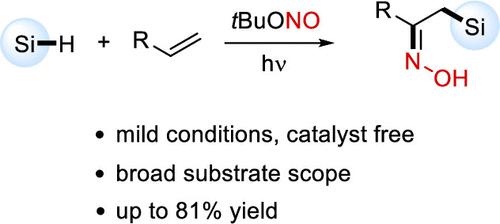
A metal-free radical 1,2-difunctionalization of activated alkenes with various silanes and tert-butyl nitrite is reported. The radical cascade occurs by light-promoted homolytic O–NO bond cleavage of tert-butyl nitrite and subsequent hydrogen atom abstraction by the alkoxyl radical from the silane. Silyl radical addition to the alkene and highly selective cross-coupling of the NO radical with the Si-adduct C-radical provide the silyl-oximation product in moderate to good yields. The reaction features good functional group tolerance and is easily scaled up.
中文翻译:

使用亚硝酸叔丁酯和硅烷对活化烯烃进行可见光介导的自由基甲硅烷基肟化
报道了用各种硅烷和亚硝酸叔丁酯对活化烯烃进行金属自由基 1,2-双官能化。自由基级联是通过光促进亚硝酸叔丁酯的均裂 O-NO 键断裂以及随后的烷氧基从硅烷中夺取氢原子而发生的。烯烃中的甲硅烷基自由基加成以及 NO 自由基与 Si 加合物 C 自由基的高度选择性交叉偶联以中等至良好的收率提供甲硅烷基肟化产物。该反应具有良好的官能团耐受性,易于放大。
更新日期:2022-11-10
中文翻译:

使用亚硝酸叔丁酯和硅烷对活化烯烃进行可见光介导的自由基甲硅烷基肟化
报道了用各种硅烷和亚硝酸叔丁酯对活化烯烃进行金属自由基 1,2-双官能化。自由基级联是通过光促进亚硝酸叔丁酯的均裂 O-NO 键断裂以及随后的烷氧基从硅烷中夺取氢原子而发生的。烯烃中的甲硅烷基自由基加成以及 NO 自由基与 Si 加合物 C 自由基的高度选择性交叉偶联以中等至良好的收率提供甲硅烷基肟化产物。该反应具有良好的官能团耐受性,易于放大。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号