Separation and Purification Technology ( IF 8.1 ) Pub Date : 2022-11-04 , DOI: 10.1016/j.seppur.2022.122527 Ruba Munir , Khuram Ali , Syed Abbas Zilqurnain Naqvi , Muhammad Aamer Maqsood , Muhammad Zeeshan Bashir , Saima Noreen
|
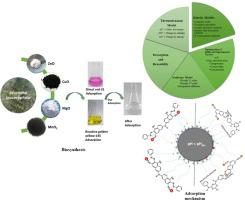
Environmental pollution by textile dye-based wastewater raises a vital call for consideration to industrialists and scientists due to its influence on the ecosystem. In this study, zinc oxide (ZnO), copper oxide (CuO), Manganese dioxide (MnO2), and Magnesium Oxide (MgO) nanoparticles were successfully synthesized from Leucaena leucocephala leaves for the removal of Reactive Golden Yellow-145 (RY-145) and Direct Red-31 (DR-31) dyes. The nanoparticles were characterized by scanning electron microscope (SEM) and Fourier Transform Infrared Spectroscopy (FTIR). Optimization of various operating parameters like initial dye concentration, pH, temperature, contact time, and dose of nanoparticles was investigated. The results showed that Leucaena leucocephala leaf-mediated ZnO, CuO, MnO2, and MgO nanoparticles presented the highest adsorption capacities for RY-145 of 47.50 (mg/g), 65.53(mg/g), 53.62 (mg/g), and 58.72 (mg/g) respectively, with optimized parameter values of pH solution, contact time, adsorbent dose, initial dye concentration, and temperature were 3, 75 min, 0.05 g/50 mL, 100 mg/L and 25°C on 124 rpm, correspondingly. DR-31 presented the highest adsorption capacities of 57.08 (mg/g), 83.47(mg/g), 67.36 (mg/g), and 72.53 (mg/g) respectively at optimized parameter values of pH solution, contact time, adsorbent dose, initial dye concentration and temperature were 2 and 3, 60min, 0.05 g/50 mL, 100 mg/L and 25°C on 124 rpm. The surfactants and electrolytes effects were also scrutinized on dyes' adsorption. The surfactants and electrolytes' existence in the aqueous media reduced the adsorption of dye because of the competition for limited binding sites. The RY-145 and DR-31 adsorbents adsorption efficiencies were found in following order; MgO (65.52% and 83.47%) > MnO2 (58.72% and 72.53%) > CuO (53.62% and 67.36%) > ZnO (47.50% and 57.08%). The adsorption-based dye data were scrutinized using various isotherms, kinetics, and thermodynamics models. The studies presented that the adsorption processes were well fitted to pseudo-second-order kinetics since correlation coefficient (R2) values ranged from 0.994 to 0.999 and Intraparticle diffusion models correlation coefficient values ranged from 0.88 to 0.97, their values were relatively near the experimental values signify the adsorption kinetics in both dyes. Both dyes adsorption on green nano-adsorbents followed a Langmuir isotherm due to decent correlation coefficient values of 0.999 with value of 59.17 (mg/g), 66.22 (mg/g), 72.99 (mg/g), and 81.30 (mg/g) respectively for DR-31 and 47.62 (mg/g), 53.763 (mg/g), 58.48 (mg/g), and 64.93 (mg/g) for RY-145 with monolayer coverage. RY-145 also followed Temkin and Doubinin Radushkevich's isotherm. The thermodynamics investigations revealed spontaneous dyes adsorption onto MgO and MnO2, ZnO is non-spontaneous in both dyes while CuO is spontaneous in DR-31 and non-spontaneous in RY-145. The maximum desorption of 78–86% was attained using the 0.2 N and 0.4 N NaOH for adsorbed RY-145 and DR-31 dye respectively. The desorption data was favorable as the Leucaena leucocephala leaf-mediated ZnO, CuO, MnO2, and MgO nanoparticles adsorbents were utilized several cycles and have stability This experimental study also determines the potential efficacy of green nano-adsorbents for the removal of dye from the real textile effluent. In addition, a phytotoxicity investigation of used green nano-adsorbents is carried out on the pea seeds, to demonstrate their sustainability from a real environmental point of view. Thus, Because of their promising efficiency, green ZnO, CuO, MnO2, and MgO nanoparticles have the potential to apply for dye adsorption from textile wastewater.
中文翻译:

Leucaena Leucocephala 叶介导的 ZnO、CuO、MnO2 和 MgO 基纳米吸附剂的生物合成,用于从纺织废水中去除活性金黄 145 (RY-145) 和直接红 31 (DR-31) 染料,再用于农业用途
纺织染料废水造成的环境污染因其对生态系统的影响而引起了工业家和科学家的重视。在这项研究中,从银合欢叶中成功合成了氧化锌 (ZnO)、氧化铜 (CuO)、二氧化锰 (MnO 2 ) 和氧化镁 (MgO) 纳米颗粒,用于去除活性金黄 145 (RY-145) ) 和 Direct Red-31 (DR-31) 染料。通过扫描电子显微镜(SEM)和傅里叶变换红外光谱(FTIR)对纳米颗粒进行表征。研究了各种操作参数的优化,例如初始染料浓度、pH、温度、接触时间和纳米颗粒剂量。结果表明,银合欢叶介导的 ZnO、CuO、MnO 2和 MgO 纳米颗粒对 RY-145 的吸附容量最高,分别为 47.50 (mg/g)、65.53(mg/g)、53.62 (mg/g) 和 58.72 (mg/g) ) 分别优化后的 pH 溶液参数值、接触时间、吸附剂剂量、初始染料浓度和温度分别为 3、75 分钟、0.05 g/50 mL、100 mg/L 和 25° C,转速为 124 rpm。在优化的 pH 溶液、接触时间、吸附剂参数值下,DR-31 的吸附容量最高,分别为 57.08 (mg/g)、83.47(mg/g)、67.36 (mg/g) 和 72.53 (mg/g)剂量、初始染料浓度和温度分别为 2 和 3、60 分钟、0.05 g/50 mL、100 mg/L 和 25° C转速为 124 转。还检查了表面活性剂和电解质对染料吸附的影响。由于对有限结合位点的竞争,水介质中的表面活性剂和电解质的存在减少了染料的吸附。RY-145 和 DR-31 吸附剂的吸附效率依次为:MgO(65.52%和83.47%)>MnO 2(58.72%和72.53%)>CuO(53.62%和67.36%)>ZnO(47.50%和57.08%)。使用各种等温线、动力学和热力学模型仔细检查了基于吸附的染料数据。研究表明,由于相关系数(R 2) 值范围为 0.994 至 0.999,颗粒内扩散模型相关系数值范围为 0.88 至 0.97,它们的值相对接近实验值,表明两种染料的吸附动力学。两种染料在绿色纳米吸附剂上的吸附遵循朗缪尔等温线,因为相关系数值为 0.999,其值为 59.17 (mg/g)、66.22 (mg/g)、72.99 (mg/g) 和 81.30 (mg/g) ) 分别为 DR-31 和 47.62 (mg/g)、53.763 (mg/g)、58.48 (mg/g) 和 RY-145 的单层覆盖率 64.93 (mg/g)。RY-145 也遵循 Temkin 和 Doubinin Radushkevich 的等温线。热力学研究表明染料自发吸附到 MgO 和 MnO 2 上,ZnO 在两种染料中都是非自发的,而 CuO 在 DR-31 中是自发的,而在 RY-145 中是非自发的。分别使用 0.2 N 和 0.4 N NaOH 对吸附的 RY-145 和 DR-31 染料进行 78-86% 的最大解吸。解吸数据是有利的,因为银合欢叶介导的 ZnO、CuO、MnO 2和 MgO 纳米颗粒吸附剂被使用了几个循环并且具有稳定性。该实验研究还确定了绿色纳米吸附剂从染料中去除染料的潜在功效真正的纺织废水。此外,对豌豆种子使用的绿色纳米吸附剂进行了植物毒性研究,以从真实的环境角度证明它们的可持续性。因此,由于它们有希望的效率,绿色 ZnO、CuO、MnO2 , MgO 纳米粒子有可能应用于纺织废水中的染料吸附。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号