Immunity ( IF 25.5 ) Pub Date : 2022-11-01 , DOI: 10.1016/j.immuni.2022.10.004 Zsolt Czimmerer 1 , Laszlo Halasz 2 , Bence Daniel 2 , Zsofia Varga 3 , Krisztian Bene 3 , Apolka Domokos 4 , Marten Hoeksema 5 , Zeyang Shen 6 , Wilhelm K Berger 2 , Timea Cseh 3 , Karoly Jambrovics 3 , Zsuzsanna Kolostyak 4 , Ferenc Fenyvesi 7 , Judit Varadi 7 , Szilard Poliska 3 , Gyorgy Hajas 8 , Istvan Szatmari 3 , Christopher K Glass 9 , Attila Bacsi 8 , Laszlo Nagy 10
|
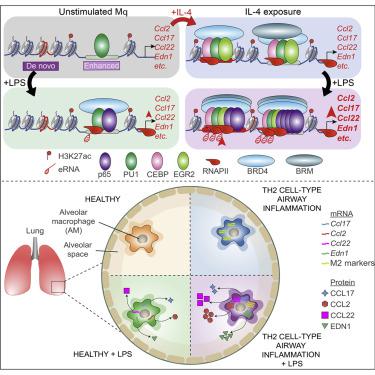
Prior exposure to microenvironmental signals could fundamentally change the response of macrophages to subsequent stimuli. It is believed that T helper-2 (Th2)-cell-type cytokine interleukin-4 (IL-4) and Toll-like receptor (TLR) ligand-activated transcriptional programs mutually antagonize each other, and no remarkable convergence has been identified between them. In contrast, here, we show that IL-4-polarized macrophages established a hyperinflammatory gene expression program upon lipopolysaccharide (LPS) exposure. This phenomenon, which we termed extended synergy, was supported by IL-4-directed epigenomic remodeling, LPS-activated NF-κB-p65 cistrome expansion, and increased enhancer activity. The EGR2 transcription factor contributed to the extended synergy in a macrophage-subtype-specific manner. Consequently, the previously alternatively polarized macrophages produced increased amounts of immune-modulatory factors both in vitro and in vivo in a murine Th2 cell-type airway inflammation model upon LPS exposure. Our findings establish that IL-4-induced epigenetic reprogramming is responsible for the development of inflammatory hyperresponsiveness to TLR activation and contributes to lung pathologies.
中文翻译:

IL-4 极化巨噬细胞的表观遗传状态能够促进炎症顺反性扩张并延长对 TLR 配体的协同反应
先前暴露于微环境信号可能从根本上改变巨噬细胞对后续刺激的反应。据认为,T 辅助细胞 2 (Th2) 细胞型细胞因子白细胞介素 4 (IL-4) 和 Toll 样受体 (TLR) 配体激活的转录程序相互拮抗,并且尚未发现两者之间存在显着的趋同。他们。相比之下,在这里,我们表明 IL-4 极化的巨噬细胞在脂多糖 (LPS) 暴露后建立了超炎症基因表达程序。我们将这种现象称为扩展协同作用,它得到了 IL-4 指导的表观基因组重塑、LPS 激活的 NF-κB-p65 顺反子扩展和增强子活性增加的支持。 EGR2转录因子以巨噬细胞亚型特异性的方式促进了扩展的协同作用。因此,在暴露于 LPS 的小鼠 Th2 细胞型气道炎症模型中,先前交替极化的巨噬细胞在体外和体内产生的免疫调节因子数量增加。我们的研究结果表明,IL-4 诱导的表观遗传重编程是 TLR 激活炎症高反应性的发生的原因,并导致肺部病理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号