Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-10-04 , DOI: 10.1016/j.apcatb.2022.122026 Jaesung Kim , Yu Jin Kim , Matthew Ferree , Seval Gunduz , Anne C. Co , Minkyu Kim , Umit S. Ozkan
|
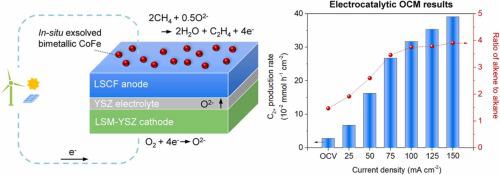
This study presents in-situ reduction of lanthanum strontium cobalt ferrite (LSCF) perovskite as an effective method for modifying its surface properties and enhancing its electrocatalytic reactivity for oxidative coupling of methane (OCM). The evolution of hetero-phases during the reduction of LSCF resulted in CoFe nanoparticles being formed at the surface. The in-situ reduced LSCF cell for OCM could be operated in either an ion pump or a fuel cell mode. High selectivity of 63% and 10.2% were reported for C2+ hydrocarbons and C3H6, respectively. DFT calculations on LSCF and CoFe revealed that the high selectivity of C2+ hydrocarbons on the LSCF primarily stems from the presence of CoFe nanoparticles. In-situ DRIFTS conducted under CH4 proved that complete oxidation of CH4 can be effectively inhibited by reducing LSCF, and control of oxygen supply is an important parameter for selective conversion of CH4 to higher order hydrocarbon.
中文翻译:

(La,Sr)FeO3钙钛矿上双金属CoFe纳米粒子的原位脱溶:其对甲烷电催化氧化耦合的影响
本研究提出了镧锶钴铁氧体 (LSCF) 钙钛矿的原位还原,作为改变其表面性质和提高其对甲烷氧化偶联 (OCM) 的电催化反应性的有效方法。LSCF 还原过程中异相的演变导致在表面形成 CoFe 纳米颗粒。用于 OCM的原位还原 LSCF 电池可以在离子泵或燃料电池模式下运行。据报道,C 2+烃和 C 3 H 6的选择性分别为 63% 和 10.2% 。对 LSCF 和 CoFe 的 DFT 计算表明,C 2+的高选择性LSCF 上的碳氢化合物主要源于 CoFe 纳米颗粒的存在。在CH 4下进行的原位DRIFTS证明,减少LSCF可以有效抑制CH 4的完全氧化,控制供氧是CH 4选择性转化为高级烃的重要参数。






























 京公网安备 11010802027423号
京公网安备 11010802027423号