当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Conserved Histidine Residue Drives Extein Dependence in an Enhanced Atypically Split Intein
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-04 , DOI: 10.1021/jacs.2c08985 Giridhar Sekar 1 , Adam J Stevens 2 , Anahita Z Mostafavi 2 , Pulikallu Sashi 1 , Tom W Muir 2 , David Cowburn 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-04 , DOI: 10.1021/jacs.2c08985 Giridhar Sekar 1 , Adam J Stevens 2 , Anahita Z Mostafavi 2 , Pulikallu Sashi 1 , Tom W Muir 2 , David Cowburn 1
Affiliation
|
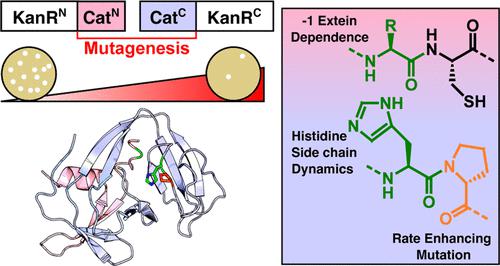
Split intein-mediated protein trans-splicing (PTS) is widely applied in chemical biology and biotechnology to carry out traceless and specific protein ligation. However, the external residues immediately flanking the intein (exteins) can reduce the splicing rate, thereby limiting certain applications of PTS. Splicing by a recently developed intein with atypical split architecture (“Cat”) exhibits a stark dependence on the sequence of its N-terminal extein residues. Here, we further developed Cat using error-prone polymerase chain reaction (PCR) and a cell-based selection assay to produce Cat*, which exhibits greatly enhanced PTS activity in the presence of unfavorable N-extein residues. We then applied solution nuclear magnetic resonance spectroscopy and molecular dynamics simulations to explore how the dynamics of a conserved B-block histidine residue (His78) contribute to this extein dependence. The enhanced extein tolerance of Cat* reported here should expand the applicability of atypically split inteins, and the mechanism highlights common principles that contribute to extein dependence.
中文翻译:

保守的组氨酸残基驱动增强型非典型分裂内含肽的外显肽依赖性
分裂内含肽介导的蛋白质转拼(PTS)广泛应用于化学生物学和生物技术中,以进行无痕且特异性的蛋白质连接。然而,紧邻内含肽(exteins)两侧的外部残基会降低剪接率,从而限制了PTS的某些应用。最近开发的具有非典型分裂结构(“Cat”)的内含肽的剪接表现出对其 N 端外显肽残基序列的完全依赖性。在这里,我们使用易错聚合酶链式反应 (PCR) 和基于细胞的选择测定进一步开发了 Cat,以产生 Cat*,它在不利的 N-外显子残基存在下表现出大大增强的 PTS 活性。然后,我们应用溶液核磁共振波谱和分子动力学模拟来探索保守 B 区组氨酸残基 (His 78 ) 的动力学如何促成这种外显子依赖性。这里报道的 Cat* 增强的外显子耐受性应该会扩大非典型分裂内含子的适用性,并且该机制突出了有助于外显子依赖的共同原理。
更新日期:2022-10-04
中文翻译:

保守的组氨酸残基驱动增强型非典型分裂内含肽的外显肽依赖性
分裂内含肽介导的蛋白质转拼(PTS)广泛应用于化学生物学和生物技术中,以进行无痕且特异性的蛋白质连接。然而,紧邻内含肽(exteins)两侧的外部残基会降低剪接率,从而限制了PTS的某些应用。最近开发的具有非典型分裂结构(“Cat”)的内含肽的剪接表现出对其 N 端外显肽残基序列的完全依赖性。在这里,我们使用易错聚合酶链式反应 (PCR) 和基于细胞的选择测定进一步开发了 Cat,以产生 Cat*,它在不利的 N-外显子残基存在下表现出大大增强的 PTS 活性。然后,我们应用溶液核磁共振波谱和分子动力学模拟来探索保守 B 区组氨酸残基 (His 78 ) 的动力学如何促成这种外显子依赖性。这里报道的 Cat* 增强的外显子耐受性应该会扩大非典型分裂内含子的适用性,并且该机制突出了有助于外显子依赖的共同原理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号