当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Stabilization of Cation-Disordered Rock-Salt Cathode Materials: Coupling between a High-Ratio Inactive Ti4+ Cation and a Mn2+/Mn4+ Two-Electron Redox Pair
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-08-12 , DOI: 10.1021/acsami.2c10652 Weijian Tang 1, 2 , Afei Li 1 , Guojun Zhou 1 , Zhangxian Chen 1, 2 , Zeheng Yang 1 , Jianhui Su 2, 3 , Weixin Zhang 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-08-12 , DOI: 10.1021/acsami.2c10652 Weijian Tang 1, 2 , Afei Li 1 , Guojun Zhou 1 , Zhangxian Chen 1, 2 , Zeheng Yang 1 , Jianhui Su 2, 3 , Weixin Zhang 1, 2
Affiliation
|
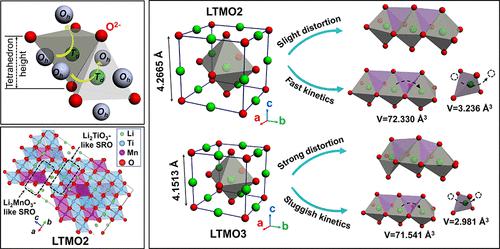
Cation-disordered rock-salt cathode materials are featured by their extraordinarily high specific capacities in lithium-ion batteries primarily contributed by anion redox reactions. Unfortunately, anion redox reactions can trigger oxygen release in this class of materials, leading to fast capacity fading and major safety concern. Despite the capability of absorbing structural distortions, high-ratio d0 transition-metal cations are considered to be unfavorable in design of a new cation-disordered rock-salt structure because of their electrochemically inactive nature. Herein, we report a new cation-disordered rock-salt compound of Li1.2Ti0.6Mn0.2O2 with the stoichiometry of Ti4+ as high as 0.6. The capacity reducing effect by the low-ratio active transition-metal center can be balanced by using a Mn2+/Mn4+ two-electron redox couple. The strengthened networks of strong Ti–O bonds greatly retard the oxygen release and improve the structural stability of cation-disordered rock-salt cathode materials. As expected, Li1.2Ti0.6Mn0.2O2 delivers significantly improved electrochemical performances and thermal stability compared to the low-ratio Ti4+ counterpart of Li1.2Ti0.4Mn0.4O2. Theoretical simulations further reveal that the improved electrochemical performances of Li1.2Ti0.6Mn0.2O2 are attributed to its lower Li+ diffusion energy barrier and enhanced unhybridized O 2p states compared to Li1.2Ti0.4Mn0.4O2. This concept might be helpful for the improvement of structural stability and electrochemical performances of other cation-disordered rock-salt metal oxide cathode materials.
中文翻译:

阳离子无序岩盐阴极材料的结构稳定:高比非活性 Ti4+ 阳离子与 Mn2+/Mn4+ 双电子氧化还原对之间的耦合
阳离子无序岩盐正极材料的特点是在主要由阴离子氧化还原反应贡献的锂离子电池中具有极高的比容量。不幸的是,阴离子氧化还原反应会在这类材料中引发氧气释放,导致容量快速衰减和重大安全问题。尽管具有吸收结构变形的能力,但高比例 d 0过渡金属阳离子由于其电化学惰性而被认为不利于设计新的阳离子无序岩盐结构。在此,我们报道了一种新的阳离子无序岩盐化合物 Li 1.2 Ti 0.6 Mn 0.2 O 2,其化学计量为 Ti 4+高达0.6。通过使用Mn 2+ /Mn 4+二电子氧化还原电对可以平衡低比率活性过渡金属中心的容量降低效果。强 Ti-O 键的强化网络极大地延缓了氧的释放,提高了阳离子无序岩盐正极材料的结构稳定性。正如预期的那样,与Li 1.2 Ti 0.4 Mn 0.4 O 2的低比例Ti 4+对应物相比,Li 1.2 Ti 0.6 Mn 0.2 O 2提供显着改善的电化学性能和热稳定性. 理论模拟进一步表明,与Li 1.2 Ti 0.4 Mn 0.4 O 2相比,Li 1.2 Ti 0.6 Mn 0.2 O 2改进的电化学性能归因于其较低的Li +扩散能垒和增强的未杂化O 2p 态。这一概念可能有助于提高其他阳离子无序岩盐金属氧化物正极材料的结构稳定性和电化学性能。
更新日期:2022-08-12
中文翻译:

阳离子无序岩盐阴极材料的结构稳定:高比非活性 Ti4+ 阳离子与 Mn2+/Mn4+ 双电子氧化还原对之间的耦合
阳离子无序岩盐正极材料的特点是在主要由阴离子氧化还原反应贡献的锂离子电池中具有极高的比容量。不幸的是,阴离子氧化还原反应会在这类材料中引发氧气释放,导致容量快速衰减和重大安全问题。尽管具有吸收结构变形的能力,但高比例 d 0过渡金属阳离子由于其电化学惰性而被认为不利于设计新的阳离子无序岩盐结构。在此,我们报道了一种新的阳离子无序岩盐化合物 Li 1.2 Ti 0.6 Mn 0.2 O 2,其化学计量为 Ti 4+高达0.6。通过使用Mn 2+ /Mn 4+二电子氧化还原电对可以平衡低比率活性过渡金属中心的容量降低效果。强 Ti-O 键的强化网络极大地延缓了氧的释放,提高了阳离子无序岩盐正极材料的结构稳定性。正如预期的那样,与Li 1.2 Ti 0.4 Mn 0.4 O 2的低比例Ti 4+对应物相比,Li 1.2 Ti 0.6 Mn 0.2 O 2提供显着改善的电化学性能和热稳定性. 理论模拟进一步表明,与Li 1.2 Ti 0.4 Mn 0.4 O 2相比,Li 1.2 Ti 0.6 Mn 0.2 O 2改进的电化学性能归因于其较低的Li +扩散能垒和增强的未杂化O 2p 态。这一概念可能有助于提高其他阳离子无序岩盐金属氧化物正极材料的结构稳定性和电化学性能。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号