当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development, Optimization, and In Vivo Validation of New Imidazopyridine Chemotypes as Dual TLR7/TLR9 Antagonists through Activity-Directed Sequential Incorporation of Relevant Structural Subunits
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-08-12 , DOI: 10.1021/acs.jmedchem.2c00386 Nirmal Das 1, 2 , Purbita Bandopadhyay 2, 3 , Swarnali Roy 1 , Bishnu Prasad Sinha 2, 3 , Uddipta Ghosh Dastidar 1, 2 , Oindrila Rahaman 3 , Sourav Pal 1, 2 , Dipyaman Ganguly 2, 3 , Arindam Talukdar 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-08-12 , DOI: 10.1021/acs.jmedchem.2c00386 Nirmal Das 1, 2 , Purbita Bandopadhyay 2, 3 , Swarnali Roy 1 , Bishnu Prasad Sinha 2, 3 , Uddipta Ghosh Dastidar 1, 2 , Oindrila Rahaman 3 , Sourav Pal 1, 2 , Dipyaman Ganguly 2, 3 , Arindam Talukdar 1, 2
Affiliation
|
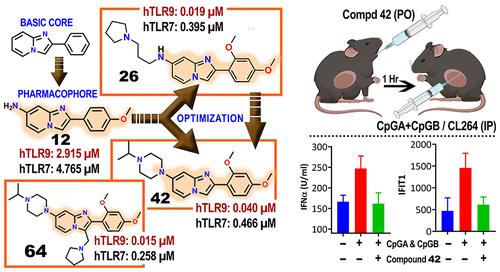
Undesirable activation of endosomal toll-like receptors TLR7 and TLR9 present in specific immune cells in response to host-derived ligands is implicated in several autoimmune diseases and other contexts of autoreactive inflammation, making them important therapeutic targets. We report a drug development strategy identifying a new chemotype for incorporating relevant structural subunits into the basic imidazopyridine core deemed necessary for potent TLR7 and TLR9 dual antagonism. We established minimal pharmacophoric features in the core followed by hit-to-lead optimization, guided by in vitro and in vivo biological assays and ADME. A ligand–receptor binding hypothesis was proposed, and selectivity studies against TLR8 were performed. Oral absorption and efficacy of lead candidate 42 were established through favorable in vitro pharmacokinetics and in vivo pharmacodynamic studies, with IC50 values of 0.04 and 0.47 μM against TLR9 and TLR7, respectively. The study establishes imidazopyridine as a viable chemotype to therapeutically target TLR9 and TLR7 in relevant clinical contexts.
中文翻译:

通过相关结构亚基的活性定向顺序掺入,开发、优化和体内验证新的咪唑并吡啶化学型作为双 TLR7/TLR9 拮抗剂
响应宿主衍生的配体,存在于特定免疫细胞中的内体 toll 样受体 TLR7 和 TLR9 的不希望激活与几种自身免疫性疾病和其他自身反应性炎症有关,使其成为重要的治疗靶点。我们报告了一种药物开发策略,该策略确定了一种新的化学型,用于将相关结构亚基整合到基本的咪唑并吡啶核心中,这些核心被认为是有效的 TLR7 和 TLR9 双重拮抗所必需的。我们在体外和体内生物测定和 ADME 的指导下,在核心中建立了最小的药效团特征,然后进行了先导优化。提出了配体-受体结合假说,并进行了针对 TLR8 的选择性研究。先导候选药物42的口服吸收和疗效通过有利的体外药代动力学和体内药效学研究确定,对 TLR9 和 TLR7 的 IC 50值分别为 0.04 和 0.47 μM。该研究确立了咪唑并吡啶作为一种可行的化学类型,可在相关临床环境中治疗靶向 TLR9 和 TLR7。
更新日期:2022-08-12
中文翻译:

通过相关结构亚基的活性定向顺序掺入,开发、优化和体内验证新的咪唑并吡啶化学型作为双 TLR7/TLR9 拮抗剂
响应宿主衍生的配体,存在于特定免疫细胞中的内体 toll 样受体 TLR7 和 TLR9 的不希望激活与几种自身免疫性疾病和其他自身反应性炎症有关,使其成为重要的治疗靶点。我们报告了一种药物开发策略,该策略确定了一种新的化学型,用于将相关结构亚基整合到基本的咪唑并吡啶核心中,这些核心被认为是有效的 TLR7 和 TLR9 双重拮抗所必需的。我们在体外和体内生物测定和 ADME 的指导下,在核心中建立了最小的药效团特征,然后进行了先导优化。提出了配体-受体结合假说,并进行了针对 TLR8 的选择性研究。先导候选药物42的口服吸收和疗效通过有利的体外药代动力学和体内药效学研究确定,对 TLR9 和 TLR7 的 IC 50值分别为 0.04 和 0.47 μM。该研究确立了咪唑并吡啶作为一种可行的化学类型,可在相关临床环境中治疗靶向 TLR9 和 TLR7。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号