Biochimie ( IF 3.3 ) Pub Date : 2022-05-26 , DOI: 10.1016/j.biochi.2022.05.016 Kehinde S Ayinde 1 , Glaucia M S Pinheiro 2 , Carlos H I Ramos 2
|
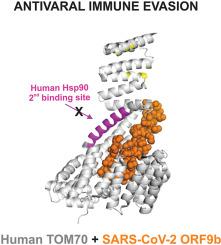
The emergence of the COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a great threat to global health. ORF9b, an important accessory protein of SARS-CoV-2, plays a critical role in the viral host interaction, targeting TOM70, a member of the mitochondrial translocase of the outer membrane complex. The assembly between ORF9b and TOM70 is implicated in disrupting mitochondrial antiviral signaling, leading to immune evasion. We describe the expression, purification, and characterization of ORF9b alone or coexpressed with the cytosolic domain of human TOM70 in E. coli. ORF9b has 97 residues and was purified as a homodimer with an molecular mass of 22 kDa as determined by SEC-MALS. Circular dichroism experiments showed that Orf9b alone exhibits a random conformation. The ORF9b-TOM70 complex characterized by CD and differential scanning calorimetry showed that the complex is folded and more thermally stable than free TOM70, indicating strong binding. Importantly, protein–protein interaction assays demonstrated that full-length human Hsp90 is capable of binding to free TOM70 but not to the ORF9b-TOM70 complex. To narrow down the nature of this inhibition, the isolated C-terminal domain of Hsp90 was also tested. These results were used to build a model of the mechanism of inhibition, in which ORF9b efficiently targets two sites of interaction between TOM70 and Hsp90. The findings showed that ORF9b complexed with TOM70 prevents the interaction with Hsp90, and this is one major explanation for SARS-CoV-2 evasion of host innate immunity via the inhibition of the interferon activation pathway.
中文翻译:

SARS-CoV-2 蛋白 ORF9b 与线粒体转位酶 TOM70 的结合可防止其与伴侣 HSP90 相互作用
由严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 引起的 COVID-19 大流行的出现,仍然对全球健康构成巨大威胁。ORF9b 是 SARS-CoV-2 的重要辅助蛋白,在病毒宿主相互作用中起着关键作用,靶向外膜复合物线粒体转位酶成员 TOM70。ORF9b 和 TOM70 之间的组装涉及破坏线粒体抗病毒信号,导致免疫逃避。我们描述了 ORF9b 单独或与大肠杆菌中人 TOM70 的胞质结构域共表达的表达、纯化和表征. ORF9b 有 97 个残基,被纯化为同型二聚体,通过 SEC-MALS 测定其分子量为 22 kDa。圆二色性实验表明,Orf9b 单独表现出随机构象。通过 CD 和差示扫描量热法表征的 ORF9b-TOM70 复合物表明,该复合物是折叠的,并且比游离 TOM70 更热稳定,表明结合力强。重要的是,蛋白质-蛋白质相互作用分析表明,全长人 Hsp90 能够结合游离 TOM70,但不能结合 ORF9b-TOM70 复合物。为了缩小这种抑制的性质,还测试了 Hsp90 的分离的 C 末端结构域。这些结果用于构建抑制机制模型,其中 ORF9b 有效靶向 TOM70 和 Hsp90 之间的两个相互作用位点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号