Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-05-13 , DOI: 10.1016/j.cej.2022.136978 Yonggyun Bae , Jongsup Hong
|
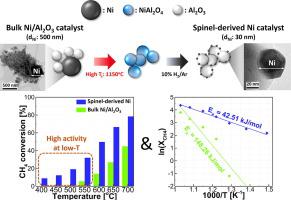
Dry reforming of methane at low temperatures of 400 ∼ 700 °C is examined by using conventional Ni/Al2O3 catalyst and spinel-derived Ni catalyst anchored on aluminum oxide through powder-based solid-state reaction. The spinel-derived Ni catalyst exhibits uniform particle distribution with a size of 10 nm (10 times smaller than the conventional Ni/Al2O3 catalyst) and the highest catalytic performance under all operating conditions with apparent activation energies of 42.51 kJ/mol for CH4 conversion and 45.39 kJ/mol for CO2 conversion. Kinetics analysis elucidates that the conventional Ni/Al2O3 catalyst changes its rate-limiting factor from diffusion limitation to kinetics limitation with decreasing temperature near 550 °C. In contrast, the spinel-derived Ni catalyst has a single rate-limiting factor of diffusion limitation at all temperatures avoiding surface kinetics limitation. In addition, it is shown that the spinel-derived Ni catalyst exhibits better stability than the conventional Ni/Al2O3 catalyst, attributed to the enhanced interaction between active Ni particles and Al2O3 support.
中文翻译:

增强 NiAl2O4 尖晶石衍生 Ni 催化剂的表面形态和催化动力学,以促进甲烷在低温下干重整,直接应用于固体氧化物燃料电池
通过使用传统的 Ni/Al 2 O 3催化剂和通过粉末固相反应锚定在氧化铝上的尖晶石衍生的 Ni 催化剂,研究了甲烷在 400 ∼ 700 °C 的低温下的干重整。尖晶石衍生的 Ni 催化剂表现出均匀的颗粒分布,粒径为 10 nm(比传统 Ni/Al 2 O 3催化剂小 10 倍),在所有操作条件下具有最高的催化性能,表观活化能为 42.51 kJ/mol CH 4转化率和CO 2转化率45.39 kJ/mol 。动力学分析表明,传统的 Ni/Al 2 O 3随着温度在 550 °C 附近降低,催化剂的限速因子从扩散限制变为动力学限制。相反,尖晶石衍生的镍催化剂在所有温度下都具有单一的扩散限制速率限制因素,避免了表面动力学限制。此外,表明尖晶石衍生的Ni催化剂比传统的Ni/Al 2 O 3催化剂表现出更好的稳定性,这归因于活性Ni颗粒与Al 2 O 3载体之间的相互作用增强。






























 京公网安备 11010802027423号
京公网安备 11010802027423号