当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (−)-3-Oxoisotaxodione
Organic Letters ( IF 4.9 ) Pub Date : 2022-03-21 , DOI: 10.1021/acs.orglett.2c00444 Samuel J Plamondon 1 , James L Gleason 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-03-21 , DOI: 10.1021/acs.orglett.2c00444 Samuel J Plamondon 1 , James L Gleason 1
Affiliation
|
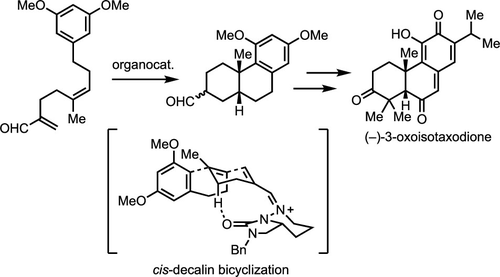
The first total synthesis of the abietaquinone methide diterpenoid (−)-3-oxoisotaxodione is reported. The key enabling step is the use of a chiral bicyclic hydrazide as an organocatalyst for the enantioselective polyene cyclization of a (Z)-polyene substrate to form the cis-decalin core of the natural product. The α-oxo-para-quinone methide unit is formed by a two-step oxidation from a phenol, enabling an efficient synthesis of the natural product.
中文翻译:

(-)-3-氧代异紫杉二酮的全合成
报道了松香醌甲基二萜类 (-)-3-氧代异紫杉二酮的首次全合成。关键的实现步骤是使用手性双环酰肼作为有机催化剂,用于 ( Z )-多烯底物的对映选择性多烯环化以形成天然产物的顺式-萘烷核心。α-氧代-对-醌甲基化物单元由苯酚通过两步氧化形成,从而能够有效合成天然产物。
更新日期:2022-03-21
中文翻译:

(-)-3-氧代异紫杉二酮的全合成
报道了松香醌甲基二萜类 (-)-3-氧代异紫杉二酮的首次全合成。关键的实现步骤是使用手性双环酰肼作为有机催化剂,用于 ( Z )-多烯底物的对映选择性多烯环化以形成天然产物的顺式-萘烷核心。α-氧代-对-醌甲基化物单元由苯酚通过两步氧化形成,从而能够有效合成天然产物。





























 京公网安备 11010802027423号
京公网安备 11010802027423号