Chinese Journal of Chemical Engineering Pub Date : 2022-02-03 , DOI: 10.1016/j.cjche.2021.12.026 Xuan Gao 1 , Zhihui Li 1, 2 , Dongsheng Zhang 1 , Xinqiang Zhao 1 , Yanji Wang 1
|
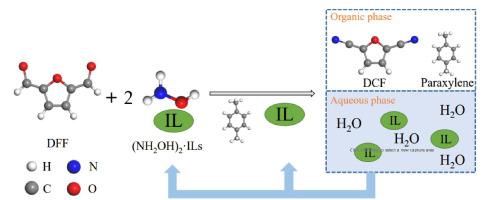
2,5-Dicyanofuran (DCF) is an important biomass-derived platform compound primarily used to prepare bio-based adiponitrile, which is the key precursor for the synthesis of nylon 66 and 1,6-hexanediisocyanate (HDI). In this study, one-pot, green and safe synthesis of DCF from 2,5-diformylfuran (DFF) and hydroxylamine ionic liquid salts was proposed. Eco-friendly hydroxylamine ionic liquid salts were used as the nitrogen source. Ionic liquid exhibited three-fold function of co-solvent, catalysis and phase separation. The conversion of DFF and yield of DCF reached 100% under the following optimum reaction conditions: temperature of 120 ℃ for 70 min, volume ratio of paraxylene: [HSO3-b-Py]·HSO4 of 2:1, and molar ratio of DFF:(NH2OH)2·[HSO3-b-Py]·HSO4 of 1:1.5. The reaction mechanism for the synthesis of DCF was proposed, and the kinetic model was established. The reaction order with respect to DFF and intermediate product 2,5-diformylfuran dioxime (DFFD) was 1.06 and 0.16, and the reaction activation energy was 64.07 kJ·mol−1and 59.37 kJ·mol−1 respectively. After the reaction, the ionic liquid was easy to separate, recover and recycle.
中文翻译:

羟胺离子液体盐存在下2,5-二氰基呋喃的合成及动力学
2,5-二氰基呋喃 (DCF) 是一种重要的生物质衍生平台化合物,主要用于制备生物基己二腈,是合成尼龙 66 和 1,6-己二异氰酸酯 (HDI) 的关键前体。本研究提出以2,5-二甲酰基呋喃(DFF)和羟胺离子液体盐为原料一锅法、绿色、安全地合成DCF。使用环保的羟胺离子液体盐作为氮源。离子液体表现出共溶剂、催化和相分离三重功能。在以下最佳反应条件下,DFF的转化率和DCF的收率达到100%:温度120℃,反应时间70 min,对二甲苯体积比:[HSO 3 -b-Py]·HSO 4为2:1,摩尔比DFF:(NH 2 OH) 2 ·[HSO3 -b-Py]·HSO 4为 1:1.5。提出了合成DCF的反应机理,建立了动力学模型。DFF和中间产物2,5-二甲酰基呋喃二肟(DFFD)的反应级数为1.06和0.16,反应活化能分别为64.07 kJ·mol -1和59.37 kJ·mol -1。反应后离子液体易于分离、回收和循环利用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号