当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CuCl2·2H2O/TBHP mediated synthesis of β-enaminones via coupling reaction of vinyl azides with aldehydes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-02-07 , DOI: 10.1039/d1ob02479e Yaohong Zhang 1 , Mengqiang Luo 1, 2 , Yichan Zhang 3 , Kai Cheng 1 , Yong Li 1 , Chenze Qi 1 , Runpu Shen 2 , Hai Wang 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-02-07 , DOI: 10.1039/d1ob02479e Yaohong Zhang 1 , Mengqiang Luo 1, 2 , Yichan Zhang 3 , Kai Cheng 1 , Yong Li 1 , Chenze Qi 1 , Runpu Shen 2 , Hai Wang 1
Affiliation
|
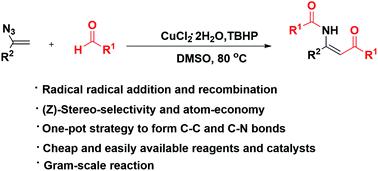
A facile and efficient oxidative functionalization of vinyl azides with aldehydes furnishing a diverse array of β-acylated enaminones was developed. The cross coupling was accomplished in the presence of CuCl2·2H2O/TBHP and produced the desired β-acylated enaminones in a (Z)-stereo-selective and atom-economic manner, which make this protocol particularly attractive. In the transformation, the new C–C and C–N bonds were formed via a one-pot strategy including the process of radical addition and recombination.
中文翻译:

CuCl2·2H2O/TBHP介导的乙烯基叠氮化物与醛的偶联反应合成β-烯胺酮
开发了一种简便有效的乙烯基叠氮化物与醛的氧化官能化,提供了多种 β-酰化烯胺酮。交叉偶联是在 CuCl 2 ·2H 2 O/TBHP 存在下完成的,并以 ( Z )-立体选择性和原子经济的方式产生所需的 β-酰化烯胺酮,这使得该协议特别有吸引力。在转化过程中,新的 C-C 和 C-N 键是通过一锅法形成的,包括自由基加成和重组过程。
更新日期:2022-02-07
中文翻译:

CuCl2·2H2O/TBHP介导的乙烯基叠氮化物与醛的偶联反应合成β-烯胺酮
开发了一种简便有效的乙烯基叠氮化物与醛的氧化官能化,提供了多种 β-酰化烯胺酮。交叉偶联是在 CuCl 2 ·2H 2 O/TBHP 存在下完成的,并以 ( Z )-立体选择性和原子经济的方式产生所需的 β-酰化烯胺酮,这使得该协议特别有吸引力。在转化过程中,新的 C-C 和 C-N 键是通过一锅法形成的,包括自由基加成和重组过程。





























 京公网安备 11010802027423号
京公网安备 11010802027423号