Ultrasonics Sonochemistry ( IF 8.7 ) Pub Date : 2022-01-21 , DOI: 10.1016/j.ultsonch.2022.105929
Rija Ansari 1 , Deepak M Kirpalani 1
|
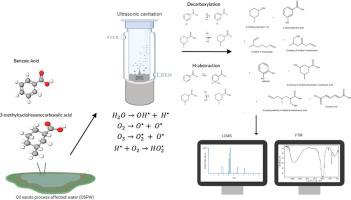
Propelled by enormous increase in demand for fuel sources, Canadian oil sands are becoming increasingly important as a fuel source due to their abundance and upgrading capability. However, extraction of bitumen, a high acid crude (HAC) oil, requires 2–3 units of water per unit of oil resulting in naphthenic acid (NA)-rich oil sands process affected water (OSPW) collected in effluent ponds. This study illustrates the role of sonochemistry in the accelerated degradation through H-abstraction and subsequent decarboxylation of aromatic and alicyclic naphthenic acid model compounds. Benzoic acid and 3-methylcyclohexane carboxylic acid were selected as model NA compounds to investigate the mechanism of hydroxyl radical (OH•) initiated carboxylic acid degradation in 378 KHz sonochemical reactor. Established FTIR methods with low resolution LCMS spectroscopy confirmation were applied to determine the extent of carboxylic acid degradation and identify the formation of products. FTIR monitoring showed a non-linear degradation of carboxylic acids with formation of many intermediates highlighting the shift from cyclic carboxylic acids to cyclic alcohols during BA degradation. Subsequent decrease in carboxylic acid groups signifies scission of cyclic structures before complete mineralization. This is confirmed with the LCMS identification of products such as: 3-hydroxybenzoic acid and phenol. This study postulated new breakdown pathways for degradation of benzoic acid with complete mineralization at a sonochemical reaction time (SRT) of 4 h. A radical quenching process was also inferred through the formation of conglomerates during sonochemical degradation of BA. Extension of the study to 3-methylcyclohexane carboxylic acid (3mCHA) shows similar non-linearity with an increase in carboxylic acid groups indicating H-abstraction followed by ring-opened compounds. However, due to the complex nature of 3mCHA’s ring-opened compounds, complete mineralization is not achieved. The putative role of sonochemistry is a promising and sustainable degradation method for mitigating NAs in OSPW, but sonication periods need to be considered carefully to ensure adequate mineralization of their constituents and combinatorial methods with other advanced oxidation methods may be needed to enhance industrial application.
In Part II, an in silico screening approach using first principles is reported to identify the breakdown of the organic compounds and determine molecular rates of reaction to confirm the mechanistic origins of the compounds formed.
中文翻译:

深入了解超声促进油砂过程中环烷酸化合物降解影响水。第一部分:加速芳族和脂环族化合物的 H-抽象和脱羧
在对燃料源需求的巨大增长的推动下,加拿大油砂因其丰富和升级能力而变得越来越重要。然而,沥青(一种高酸原油 (HAC) 油)的提取每单位油需要 2-3 个单位的水,导致在污水池中收集的富含环烷酸 (NA) 的油砂工艺影响水 (OSPW)。这项研究说明了声化学在通过 H 提取和随后的芳香族和脂环族环烷酸模型化合物的脱羧加速降解中的作用。选择苯甲酸和3-甲基环己烷羧酸作为模型NA化合物,研究羟基自由基(OH •) 在 378 KHz 声化学反应器中引发羧酸降解。应用已建立的具有低分辨率 LCMS 光谱确认的 FTIR 方法来确定羧酸降解的程度并确定产物的形成。FTIR 监测显示羧酸的非线性降解,形成了许多中间体,突出了 BA 降解过程中从环状羧酸到环状醇的转变。随后羧酸基团的减少意味着在完全矿化之前环状结构的断裂。这通过 LCMS 对以下产品的鉴定得到证实:3-羟基苯甲酸和苯酚。该研究假设在 4 小时的声化学反应时间 (SRT) 内完全矿化的苯甲酸降解的新分解途径。还通过在 BA 的声化学降解过程中形成团块来推断自由基猝灭过程。将研究扩展到 3-甲基环己烷羧酸 (3mCHA) 显示出类似的非线性,随着羧酸基团的增加,表明 H 抽象随后是开环化合物。然而,由于 3mCHA 开环化合物的复杂性,无法实现完全矿化。声化学的假定作用是减轻 OSPW 中 NAs 的一种有前途且可持续的降解方法,但需要仔细考虑超声处理时间以确保其成分的充分矿化,并且可能需要将方法与其他高级氧化方法相结合以增强工业应用。
在第二部分中,报告了使用第一原理的计算机筛选方法来识别有机化合物的分解并确定反应的分子速率,以确认所形成化合物的机理来源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号