Nano Today ( IF 13.2 ) Pub Date : 2022-01-18 , DOI: 10.1016/j.nantod.2022.101395 Juqun Xi 1 , Yanqiu Wang 1 , Xuejiao Gao 2 , Yaling Huang 1 , Jie Chen 1 , Yong Chen 3 , Lei Fan 4 , Lizeng Gao 5
|
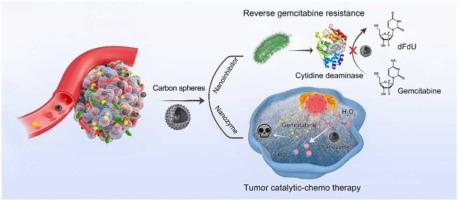
Emerging evidence demonstrates that intratumor bacteria promote tumor resistance to gemcitabine chemotherapy. The underlying mechanism is relate to intratumor bacteria expressing a specific cytidine deaminase (CDD) which metabolizes gemcitabine into its inactive form. Therefore, to overcome tumor resistance to gemcitabine, the CDD activity of intratumor bacteria must be restricted during chemotherapy. Herein, we developed an antitumor strategy by utilizing the dual functions of nitrogen-doped carbon nanospheres (N-CSs), i.e., as nanoinhibitors of CDD to overcome bacteria-mediated gemcitabine resistance and as nanozymes to integrate tumor catalytic therapy with chemotherapy. We found that the nitrogen-doped graphitized structure of the N-CSs could competitively bind to the active center of CDD and thus prevented gemcitabine metabolism. In addition, nitrogen doping endowed the N-CSs with peroxidase-like activity that generates •OH radicals for tumor catalytic therapy. Furthermore, in mouse cancer models with intratumor bacteria, the N-CSs successfully reversed bacterial CDD-induced gemcitabine resistance and restored tumor susceptibility to gemcitabine. The synergistic effects of catalytic therapy and chemotherapy for tumor treatment were achieved by using single N-CSs with dual nanozymatic and nanoinhibitory functions. This work provides a promising approach to overcome tumor drug-resistance induced by intratumor bacteria and synergize gemcitabine chemotherapy with nanozyme-mediated catalytic therapy for tumor treatment.
中文翻译:

用碳纳米酶逆转肿瘤内细菌诱导的吉西他滨耐药性以增强肿瘤催化化疗
新出现的证据表明,肿瘤内细菌会促进肿瘤对吉西他滨化疗的耐药性。潜在的机制与表达特定胞苷脱氨酶 (CDD) 的肿瘤内细菌有关,该胞苷脱氨酶 (CDD) 将吉西他滨代谢成无活性形式。因此,为了克服肿瘤对吉西他滨的耐药性,化疗期间必须限制肿瘤内细菌的CDD活性。在此,我们利用氮掺杂碳纳米球 (N-CSs) 的双重功能开发了一种抗肿瘤策略,即作为 CDD 的纳米抑制剂来克服细菌介导的吉西他滨耐药性,以及作为纳米酶将肿瘤催化治疗与化学疗法相结合。我们发现 N-CSs 的氮掺杂石墨化结构可以竞争性地结合 CDD 的活性中心,从而阻止吉西他滨代谢。此外,氮掺杂赋予 N-CSs 类似过氧化物酶的活性,产生用于肿瘤催化治疗的•OH 自由基。此外,在具有肿瘤内细菌的小鼠癌症模型中,N-CSs 成功地逆转了细菌 CDD 诱导的吉西他滨耐药性并恢复了肿瘤对吉西他滨的敏感性。通过使用具有双重纳米酶和纳米抑制功能的单个 N-CS,实现了催化疗法和化学疗法对肿瘤治疗的协同作用。这项工作提供了一种有前景的方法来克服由肿瘤内细菌引起的肿瘤耐药性,并将吉西他滨化疗与纳米酶介导的催化治疗协同治疗肿瘤。在具有瘤内细菌的小鼠癌症模型中,N-CSs 成功地逆转了细菌 CDD 诱导的吉西他滨耐药性并恢复了肿瘤对吉西他滨的敏感性。通过使用具有双重纳米酶和纳米抑制功能的单个 N-CS,实现了催化疗法和化学疗法对肿瘤治疗的协同作用。这项工作提供了一种有前景的方法来克服由肿瘤内细菌引起的肿瘤耐药性,并将吉西他滨化疗与纳米酶介导的催化治疗协同治疗肿瘤。在具有瘤内细菌的小鼠癌症模型中,N-CSs 成功地逆转了细菌 CDD 诱导的吉西他滨耐药性并恢复了肿瘤对吉西他滨的敏感性。通过使用具有双重纳米酶和纳米抑制功能的单个 N-CS,实现了催化疗法和化学疗法对肿瘤治疗的协同作用。这项工作提供了一种有前景的方法来克服由肿瘤内细菌引起的肿瘤耐药性,并将吉西他滨化疗与纳米酶介导的催化治疗协同治疗肿瘤。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号