Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Abundance and Metabolism Disruptions of Intratumoral Microbiota by Chemical and Physical Actions Unfreeze Tumor Treatment Resistance
Advanced Science ( IF 14.3 ) Pub Date : 2022-01-17 , DOI: 10.1002/advs.202105523 Fanlei Kong 1, 2 , Chao Fang 1, 3 , Yan Zhang 1 , Lixia Duan 1, 3 , Dou Du 1 , Guang Xu 1 , Xiaolong Li 1 , Hongyan Li 1 , Yifei Yin 1 , Huixiong Xu 1 , Kun Zhang 1, 3
Advanced Science ( IF 14.3 ) Pub Date : 2022-01-17 , DOI: 10.1002/advs.202105523 Fanlei Kong 1, 2 , Chao Fang 1, 3 , Yan Zhang 1 , Lixia Duan 1, 3 , Dou Du 1 , Guang Xu 1 , Xiaolong Li 1 , Hongyan Li 1 , Yifei Yin 1 , Huixiong Xu 1 , Kun Zhang 1, 3
Affiliation
|
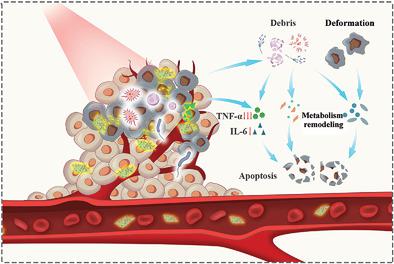
Intratumoral or intestinal microbiota correlates with tumorigenesis and progression, and microbiota regulation for reinforcing various anti-tumor approaches is of significant importance, which, however, suffers from no precise regulation method and unclear underlying mechanism. Herein, a microbiome metabolism-engineered phototherapy strategy is established, wherein Nb2C/Au nanocomposite and the corresponding phototherapy are harnessed to realize “chemical” and “physical” bacterial regulations. Flora analysis and mass spectrometry (MS) and metabonomics combined tests demonstrate that the synergistic microbiota regulations can alter the abundance, diversity of intratumoral microbiome, and disrupt metabolic pathways of microbiome and tumor microenvironment, wherein the differential singling pathways and biosynthetic necessities or metabolites that can affect tumor progression are identified. As well, anti-TNFα is introduced to unite with bacterial regulation to synergistically mitigate bacterial-induced inflammation, which, along with the metabolism disruptions of intratumoral microbiota and tumor microenvironment, unfreezes tumor resistance and harvests significantly-intensified phototherapy-based anti-tumor outcomes against 4T1 and CT26 tumors. The clear underlying principles of microbiome-regulated tumorigenesis and the established microbiome metabolism regulation method provide distinctive insights into tumor therapy, and can be also extended to other gut microbiome-associated lesions interference.
中文翻译:

化学和物理作用对肿瘤内微生物群的丰度和代谢造成破坏,从而解冻肿瘤治疗耐药性
肿瘤内或肠道微生物群与肿瘤的发生和进展相关,微生物群调节对于增强各种抗肿瘤方法具有重要意义,但缺乏精确的调节方法和不清楚的潜在机制。在此,建立了微生物组代谢工程光疗策略,其中利用Nb 2 C/Au纳米复合材料和相应的光疗来实现“化学”和“物理”细菌调节。菌群分析、质谱(MS)和代谢组学联合测试表明,微生物群的协同调节可以改变肿瘤内微生物组的丰度和多样性,并破坏微生物组和肿瘤微环境的代谢途径,其中差异化的单一途径和生物合成必需品或代谢物可以已确定对肿瘤进展的影响。此外,引入抗TNFα与细菌调节相结合,协同减轻细菌引起的炎症,再加上瘤内微生物群和肿瘤微环境的代谢破坏,解冻肿瘤抵抗力并获得显着强化的基于光疗的抗肿瘤效果针对 4T1 和 CT26 肿瘤的结果。微生物组调节肿瘤发生的明确基本原理和已建立的微生物组代谢调节方法为肿瘤治疗提供了独特的见解,也可以扩展到其他肠道微生物组相关病变的干扰。
更新日期:2022-01-17
中文翻译:

化学和物理作用对肿瘤内微生物群的丰度和代谢造成破坏,从而解冻肿瘤治疗耐药性
肿瘤内或肠道微生物群与肿瘤的发生和进展相关,微生物群调节对于增强各种抗肿瘤方法具有重要意义,但缺乏精确的调节方法和不清楚的潜在机制。在此,建立了微生物组代谢工程光疗策略,其中利用Nb 2 C/Au纳米复合材料和相应的光疗来实现“化学”和“物理”细菌调节。菌群分析、质谱(MS)和代谢组学联合测试表明,微生物群的协同调节可以改变肿瘤内微生物组的丰度和多样性,并破坏微生物组和肿瘤微环境的代谢途径,其中差异化的单一途径和生物合成必需品或代谢物可以已确定对肿瘤进展的影响。此外,引入抗TNFα与细菌调节相结合,协同减轻细菌引起的炎症,再加上瘤内微生物群和肿瘤微环境的代谢破坏,解冻肿瘤抵抗力并获得显着强化的基于光疗的抗肿瘤效果针对 4T1 和 CT26 肿瘤的结果。微生物组调节肿瘤发生的明确基本原理和已建立的微生物组代谢调节方法为肿瘤治疗提供了独特的见解,也可以扩展到其他肠道微生物组相关病变的干扰。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号