Kidney International Reports ( IF 5.7 ) Pub Date : 2021-10-30 , DOI: 10.1016/j.ekir.2021.10.017 Michelle A Hladunewich 1 , Dan Cattran 2 , Sanjeev M Sethi 3 , Salim S Hayek 4 , Jing Li 5 , Changli Wei 5 , Sarah I Mullin 1 , Heather N Reich 2 , Jochen Reiser 5 , Fernando C Fervenza 6
|
Introduction
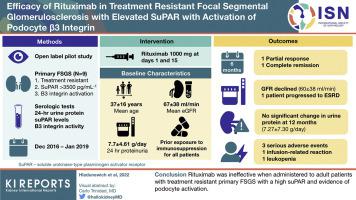
Severe, nonresponsive, primary focal segmental glomerular sclerosis (FSGS) can progress to end-stage kidney disease (ESKD) in <5 years. Soluble urokinase-type plasminogen activator receptor (suPAR) may contribute to podocyte effacement by activating podocyte β3 integrin. It has been reported as a potential permeability factor and biomarker for primary FSGS. Rituximab was found to have efficacy in case reports and small series. Whether rituximab is efficacious in patients with treatment-resistant FSGS in the context of high suPAR levels and evidence of podocyte B3 integrin activation remains unknown.
Methods
In this nonblinded, open-label pilot study, the safety and efficacy of rituximab were evaluated in treatment-resistant adult patients with primary FSGS and a suPAR level > 3500 pg/ml with evidence of β3 integrin activation. Rituximab (1 g) was given on days 1 and 15. The primary outcome was proteinuria at 12 months.
Results
Only 13 of 38 screened patients qualified for the study, of whom 9 consented to participate. The baseline proteinuria and glomerular filtration rate (GFR) levels were 7.70 ± 4.61 g/d and 67 ± 38 ml/min, respectively. A transient response at 6 months was noted in 2 patients without a parallel change in suPAR level. At 12 months, there was no statistically significant improvement in proteinuria level with all participants remaining nephrotic (7.27 ± 7.30 g/d). GFR level marginally declined to 60 ± 38 ml/min with one patient progressing to ESKD. There were 2 serious infections, an infusion-related reaction and leucopenia attributed to rituximab.
Conclusion
Rituximab was ineffective when administered to adult patients with treatment-resistant primary FSGS with a high suPAR and evidence of podocyte activation.
中文翻译:

利妥昔单抗在可溶性尿激酶型纤溶酶原激活物受体升高和足细胞 β3 整合素激活的难治性局灶节段性肾小球硬化症中的疗效
介绍
严重、无反应、原发性局灶节段性肾小球硬化 (FSGS) 可在 <5 年内发展为终末期肾病 (ESKD)。可溶性尿激酶型纤溶酶原激活物受体 (suPAR) 可能通过激活足细胞 β3 整合素促进足细胞消失。据报道,它是原发性 FSGS 的潜在渗透因子和生物标志物。在病例报告和小型系列研究中发现利妥昔单抗有效。在高 suPAR 水平和足细胞 B3 整合素激活证据的情况下,利妥昔单抗是否对难治性 FSGS 患者有效仍然未知。
方法
在这项非盲、开放标签的试点研究中,利妥昔单抗的安全性和有效性在患有原发性 FSGS 且 suPAR 水平 > 3500 pg/ml 并有 β3 整合素激活证据的难治性成年患者中进行了评估。在第 1 天和第 15 天给予利妥昔单抗 (1 g)。主要结果是 12 个月时的蛋白尿。
结果
筛选的 38 名患者中只有 13 名符合研究条件,其中 9 名同意参与。基线蛋白尿和肾小球滤过率 (GFR) 水平分别为 7.70 ± 4.61 g/d 和 67 ± 38 ml/min。2 名患者在 6 个月时出现短暂反应,suPAR 水平没有平行变化。在 12 个月时,蛋白尿水平没有统计学上的显着改善,所有参与者仍处于肾病状态 (7.27 ± 7.30 g/d)。GFR 水平略微下降至 60 ± 38 ml/min,一名患者进展为 ESKD。有 2 例严重感染、输液相关反应和归因于利妥昔单抗的白细胞减少症。
结论
对具有高 suPAR 和足细胞活化证据的难治性原发性 FSGS 成年患者使用利妥昔单抗无效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号