当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonium Metatungstate, (NH4)6[H2W12O40]: Crystallization and Thermal Behavior of Various Hydrous Species
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.cgd.1c00639 Felix Eder 1 , Matthias Weil 1 , Wolf-Dieter Schubert 2 , Christian Gierl-Mayer 3
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.cgd.1c00639 Felix Eder 1 , Matthias Weil 1 , Wolf-Dieter Schubert 2 , Christian Gierl-Mayer 3
Affiliation
|
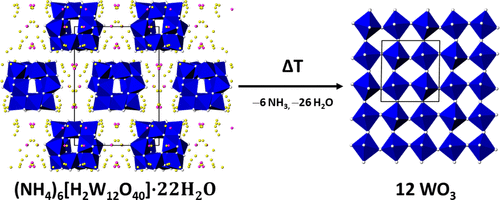
Various hydrous ammonium metatungstate phases (NH4)6[H2W12O40]·XH2O (AMT-X) have been obtained in the form of single crystals, using supersaturated solutions, antisolvent crystallization, and partial dehydration as strategies for crystal growth. On the basis of laboratory X-ray diffraction data, the crystal structures of the hydrous phases with X = 22, 12.5, 9.5, 8.5, 6, 4, and 2 as well of the cocrystals with X = 12-ethanol and X = 4-2acetone have been determined for the first time. AMT-22 forms the solid solution series (NH4)6–xKx[H2W12O40]·22H2O with the potassium salt (PMT-22) without a miscibility gap. Its tetragonal crystal structure shows a distorted cubic close-packed arrangement of the spherical α-Keggin-type [H2W12O40]6– anions with disordered ammonium N and water O atoms in the voids. The crystal structures of the other, less-hydrated AMT phases can be derived from distorted hexagonal rod packings of the α-Keggin anions, likewise with the cations and crystal water molecules in the voids. The thermal behavior of AMT-22 crystals reveals a quick dehydration, with AMT-9.5 as an intermediate and AMT-4 as a stable hydrate phase under ambient conditions. Upon further heating, the material decomposes with stepwise formation of AMT-2, AMT-1, AMT-0, and an amorphous phase, before orthorhombic WO3 forms above 400 °C. One of the commercially available AMT phases, “(NH4)6[H2W12O40]·XH2O” (with X = unspecified) has a water content of X = 4 but crystallizes in a structure other than AMT-4. Consequently, the 4-hydrate shows polymorphic behavior.
中文翻译:

偏钨酸铵,(NH4)6[H2W12O40]:各种水合物种的结晶和热行为
各种含水偏钨酸铵相 (NH 4 ) 6 [H 2 W 12 O 40 ]· X H 2 O (AMT- X ) 以单晶形式获得,使用过饱和溶液、抗溶剂结晶和部分脱水作为策略用于晶体生长。根据实验室X射线衍射数据,X = 22、12.5、9.5、8.5、6、4和2的水相的晶体结构以及X = 12-乙醇和X = 4的共晶的晶体结构-2丙酮已被首次测定。AMT-22形成固溶体系列(NH 4)6– x K x [H 2 W 12 O 40 ]·22H 2 O 与钾盐 (PMT-22) 无混溶间隙。其四方晶体结构显示球形α-Keggin型[H 2 W 12 O 40 ] 6–的扭曲立方密堆积排列空隙中具有无序铵 N 和水 O 原子的阴离子。其他水化程度较低的 AMT 相的晶体结构可以源自 α-Keggin 阴离子的扭曲六方棒堆积,同样带有空隙中的阳离子和结晶水分子。AMT-22 晶体的热行为显示出快速脱水,在环境条件下,AMT-9.5 作为中间体,AMT-4 作为稳定的水合物相。在进一步加热时,材料分解,逐步形成 AMT-2、AMT-1、AMT-0 和无定形相,然后在 400 °C 以上形成正交 WO 3。市售 AMT 相之一,“(NH 4 ) 6 [H 2 W 12 O 40 ]· X H2 O”(X = 未指定)的水含量为X = 4,但在 AMT-4 以外的结构中结晶。因此,4-水合物表现出多晶型行为。
更新日期:2021-11-03
中文翻译:

偏钨酸铵,(NH4)6[H2W12O40]:各种水合物种的结晶和热行为
各种含水偏钨酸铵相 (NH 4 ) 6 [H 2 W 12 O 40 ]· X H 2 O (AMT- X ) 以单晶形式获得,使用过饱和溶液、抗溶剂结晶和部分脱水作为策略用于晶体生长。根据实验室X射线衍射数据,X = 22、12.5、9.5、8.5、6、4和2的水相的晶体结构以及X = 12-乙醇和X = 4的共晶的晶体结构-2丙酮已被首次测定。AMT-22形成固溶体系列(NH 4)6– x K x [H 2 W 12 O 40 ]·22H 2 O 与钾盐 (PMT-22) 无混溶间隙。其四方晶体结构显示球形α-Keggin型[H 2 W 12 O 40 ] 6–的扭曲立方密堆积排列空隙中具有无序铵 N 和水 O 原子的阴离子。其他水化程度较低的 AMT 相的晶体结构可以源自 α-Keggin 阴离子的扭曲六方棒堆积,同样带有空隙中的阳离子和结晶水分子。AMT-22 晶体的热行为显示出快速脱水,在环境条件下,AMT-9.5 作为中间体,AMT-4 作为稳定的水合物相。在进一步加热时,材料分解,逐步形成 AMT-2、AMT-1、AMT-0 和无定形相,然后在 400 °C 以上形成正交 WO 3。市售 AMT 相之一,“(NH 4 ) 6 [H 2 W 12 O 40 ]· X H2 O”(X = 未指定)的水含量为X = 4,但在 AMT-4 以外的结构中结晶。因此,4-水合物表现出多晶型行为。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号