当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precise Subcellular Organelle Targeting for Boosting Endogenous-Stimuli-Mediated Tumor Therapy
Advanced Materials ( IF 27.4 ) Pub Date : 2021-10-05 , DOI: 10.1002/adma.202101572 Wenyao Zhen 1, 2 , Shangjie An 1, 2 , Shuqi Wang 1, 2 , Wenxue Hu 3 , Yujie Li 4 , Xiue Jiang 1, 2 , Jinghong Li 4
Advanced Materials ( IF 27.4 ) Pub Date : 2021-10-05 , DOI: 10.1002/adma.202101572 Wenyao Zhen 1, 2 , Shangjie An 1, 2 , Shuqi Wang 1, 2 , Wenxue Hu 3 , Yujie Li 4 , Xiue Jiang 1, 2 , Jinghong Li 4
Affiliation
|
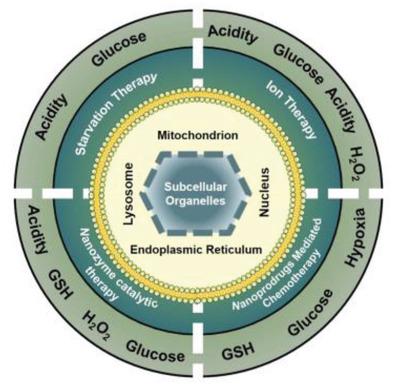
Though numerous external-stimuli-triggered tumor therapies, including phototherapy, radiotherapy, and sonodynamic therapy have made great progress in cancer therapy, the low penetration depth of the laser, safety concerns of radiation, the therapeutic resistance, and the spatio-temporal constraints of the specific equipment restrict their convenient clinical applications. What is more, the inherent physiological barriers of the tumor microenvironment (TME), including hypoxia, heterogeneity, and high expression of antioxidant molecules also restrict the efficiency of tumor therapy. As a result, the development of nanoplatforms responsive to endogenous stimuli (such as glucose, acidic pH, cellular redox events, and etc.) has attracted great attention for starvation therapy, ion therapy, prodrug-mediated chemotherapy, or enzyme-catalyzed therapy. In addition, nanomedicines can be modified by some targeted units for precisely locating in subcellular organelles and boosting the destroying of tumor tissue, decreasing the dosage of nanoagents, reducing side effects, and enhancing the therapeutic efficiency. Herein, the properties of the TME, the advantages of endogenous stimuli, and the principles of subcellular-organelle-targeted strategies will be emphasized. Some necessary considerations for the exploitation of precision medicine and clinical translation of multifunctional nanomedicines in the future are also pointed out.
中文翻译:

精确的亚细胞细胞器靶向促进内源性刺激介导的肿瘤治疗
尽管光疗、放疗、声动力疗法等多种外部刺激引发的肿瘤疗法在癌症治疗方面取得了长足的进步,但激光的穿透深度低、辐射的安全性问题、治疗的耐药性以及治疗的时空限制等问题仍然存在。特定的设备限制了其便捷的临床应用。更重要的是,肿瘤微环境(TME)固有的生理障碍,包括缺氧、异质性和抗氧化分子的高表达也限制了肿瘤治疗的效率。因此,响应内源性刺激(如葡萄糖、酸性pH、细胞氧化还原事件等)的纳米平台的开发引起了饥饿疗法、离子疗法、前药介导的化疗或酶催化疗法的极大关注。此外,纳米药物还可以通过一些靶向单元的修饰,精确定位于亚细胞器,增强对肿瘤组织的破坏,减少纳米药物的用量,减少副作用,提高治疗效率。在此,将强调 TME 的特性、内源性刺激的优势以及亚细胞器靶向策略的原理。还指出了未来多功能纳米药物在精准医疗开发和临床转化方面的一些必要考虑。
更新日期:2021-10-05
中文翻译:

精确的亚细胞细胞器靶向促进内源性刺激介导的肿瘤治疗
尽管光疗、放疗、声动力疗法等多种外部刺激引发的肿瘤疗法在癌症治疗方面取得了长足的进步,但激光的穿透深度低、辐射的安全性问题、治疗的耐药性以及治疗的时空限制等问题仍然存在。特定的设备限制了其便捷的临床应用。更重要的是,肿瘤微环境(TME)固有的生理障碍,包括缺氧、异质性和抗氧化分子的高表达也限制了肿瘤治疗的效率。因此,响应内源性刺激(如葡萄糖、酸性pH、细胞氧化还原事件等)的纳米平台的开发引起了饥饿疗法、离子疗法、前药介导的化疗或酶催化疗法的极大关注。此外,纳米药物还可以通过一些靶向单元的修饰,精确定位于亚细胞器,增强对肿瘤组织的破坏,减少纳米药物的用量,减少副作用,提高治疗效率。在此,将强调 TME 的特性、内源性刺激的优势以及亚细胞器靶向策略的原理。还指出了未来多功能纳米药物在精准医疗开发和临床转化方面的一些必要考虑。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号