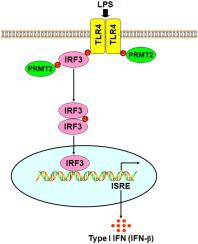
Toll 样受体 (TLR) 信号通路的正负调节之间需要平衡,以避免有害和不适当的炎症反应。尽管一些蛋白质翻译后修饰 (PTM) 如磷酸化和泛素化已被证明可以有效调节先天免疫反应,但甲基化(一种重要的 PTM)对 TLR4 信号通路的控制作用仍不清楚。在这项研究中,我们发现蛋白精氨酸甲基转移酶 1、2 和 3(PRMT1、2 和 3)分别在脂多糖(LPS)刺激后被募集到甲基化 TLR4-CD(细胞质域),但 PRMT2 对精氨酸甲基化的影响TLR4-CD 是上述三个 PRMT 中最重要的,这促使我们关注 PRMT2。在有或没有 LPS 处理的情况下,减少 PRMT2 表达会下调 TLR4 的精氨酸 (R) 甲基化水平。甲硫氨酸 115 (M115) 介导的 PRMT2 催化了 R731 和 R812 上 TLR4 的精氨酸甲基化。此外,在LPS刺激后,PRMT1、2和3分别被募集到甲基化干扰素调节因子3(IRF3),但PRMT2对IRF3精氨酸甲基化的影响在上述三种PRMT中最为显着。R812 上 TLR4 的精氨酸甲基化或 R285 上 IRF3 的精氨酸甲基化分别介导了 TLR4 和 IRF3 之间的相互作用。LPS 诱导 R285 上 IRF3 的精氨酸甲基化导致其二聚化并促进其从细胞质到细胞核的易位。此外,PRMT1或2诱导的TLR4精氨酸甲基化的增强在有或没有LPS处理的情况下增加了IRF3转录活性,而具有组氨酸112谷氨酰胺(H112Q)或甲硫氨酸115异亮氨酸(M115I)突变的PRMT2和具有精氨酸812赖氨酸(R8)突变的TLR4降低它。R812 或 PRMT2 上 TLR4 的精氨酸甲基化增强了干扰素-β (IFN-β) 的产生。我们的研究揭示了 PRMT2 和蛋白质精氨酸甲基化在增强 IFN-β 产生中的关键作用通过TLR4/IRF3 信号通路,可能为控制内毒素血症提供治疗策略。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Arginine methylation by PRMT2 promotes IFN-β production through TLR4/IRF3 signaling pathway
A balance between the positive and negative regulation of toll-like receptor (TLR) signaling pathways is required to avoid detrimental and inappropriate inflammatory responses. Although some protein post-translational modifications (PTMs) such as phosphorylation and ubiquitination have been demonstrated to potently modulate innate immune responses, the role of methylation, an important PTM, control of TLR4 signaling pathway remains unclear. In this study, we found that protein arginine methyltransferase 1, 2 and 3 (PRMT1, 2 and 3) were recruited to methylate TLR4-CD (cytoplasmic domain) after lipopolysaccharide (LPS) stimulation respectively, but the effect of PRMT2 on arginine methylation of TLR4-CD is the most significant among above three PRMTs, which prompted us to focus on PRMT2. Reduction of PRMT2 expression down-regulated arginine (R) methylation level of TLR4 with or without LPS treatment. Methionine 115 (M115) mediated PRMT2 catalyzed-arginine methylation of TLR4 on R731 and R812. Furthermore, PRMT1, 2 and 3 was recruited to methylate interferon regulatory factor 3 (IRF3) after LPS stimulation respectively, but the effect of PRMT2 on arginine methylation of IRF3 is the most significant among the above three PRMTs. Arginine methylation of TLR4 on R812 or arginine methylation of IRF3 on R285 mediated the interaction between TLR4 and IRF3 respectively. Arginine methylation of IRF3 on R285 induced by LPS led to its dimerization and promoted its translocation from the cytoplasm to the nucleus. In addition, the enhancement of arginine methylation of TLR4 induced by PRMT1 or 2 increased IRF3 transcription activity with or without LPS treatment, while PRMT2 with histidine 112 glutamine (H112Q) or methionine 115 isoleucine (M115I) mutation and TLR4 with arginine 812 lysine (R812K) mutation decreased it. Arginine methylation of TLR4 on R812 or PRMT2 enhanced interferon-β (IFN-β) production. Our study reveals a critical role for PRMT2 and protein arginine methylation in the enhancement of IFN-β production via TLR4/IRF3 signaling pathway and may provide a therapeutic strategy to control endotoxemia.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号