当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calcium-Catalyzed, Dehydrative, Ring-Opening Cyclizations of Cyclopropyl Carbinols Derived from Donor–Acceptor Cyclopropanes
Organic Letters ( IF 4.9 ) Pub Date : 2016-08-12 00:00:00 , DOI: 10.1021/acs.orglett.6b01933 Matthew J. Sandridge 1 , Stefan France 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2016-08-12 00:00:00 , DOI: 10.1021/acs.orglett.6b01933 Matthew J. Sandridge 1 , Stefan France 1, 2
Affiliation
|
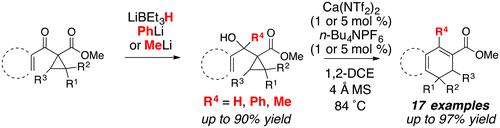
A calcium-catalyzed, dehydrative, ring-opening cyclization of (hetero)aryl cyclopropyl carbinols is reported. The cyclopropyl carbinols are prepared directly from the corresponding donor–acceptor (D–A) cyclopropanes. The calcium catalyst catalyzes the formation of putative (hetero)aryl cyclopropyl carbinyl cations that undergo ring-opening to allylcarbinyl cations. Subsequent intramolecular Friedel–Crafts reaction affords (hetero)aryl-fused cyclohexa-1,3-dienes in up to 97% yield. This approach represents the first example of catalysis for this intramolecular, dehydrative ring-opening cyclization and outperforms the previous reports using stoichiometric Lewis acids.
中文翻译:

供体-受体环丙烷衍生的环丙基甲醇的钙催化,脱水,开环环化
报道了(杂)芳基环丙基甲醇的钙催化的脱水开环环化。环丙基甲醇是直接从相应的供体-受体(D-A)环丙烷制备的。钙催化剂催化假定的(杂)芳基环丙基羧烷基阳离子的形成,该阳离子经历开环成烯丙基羰基阳离子。随后的分子内Friedel–Crafts反应可提供(杂)芳基稠合的环己-1,3-二烯,产率高达97%。这种方法代表了这种分子内脱水开环环化反应的第一个催化实例,并且优于使用化学计量路易斯酸的先前报道。
更新日期:2016-08-12
中文翻译:

供体-受体环丙烷衍生的环丙基甲醇的钙催化,脱水,开环环化
报道了(杂)芳基环丙基甲醇的钙催化的脱水开环环化。环丙基甲醇是直接从相应的供体-受体(D-A)环丙烷制备的。钙催化剂催化假定的(杂)芳基环丙基羧烷基阳离子的形成,该阳离子经历开环成烯丙基羰基阳离子。随后的分子内Friedel–Crafts反应可提供(杂)芳基稠合的环己-1,3-二烯,产率高达97%。这种方法代表了这种分子内脱水开环环化反应的第一个催化实例,并且优于使用化学计量路易斯酸的先前报道。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号