当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insulin Transdermal Delivery System for Diabetes Treatment Using a Biocompatible Ionic Liquid-Based Microemulsion
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-30 , DOI: 10.1021/acsami.1c11533
Md Rafiqul Islam 1, 2 , Shihab Uddin 1 , Md Raihan Chowdhury 1 , Rie Wakabayashi 1, 3 , Muhammad Moniruzzaman 4 , Masahiro Goto 1, 3, 5
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-30 , DOI: 10.1021/acsami.1c11533
Md Rafiqul Islam 1, 2 , Shihab Uddin 1 , Md Raihan Chowdhury 1 , Rie Wakabayashi 1, 3 , Muhammad Moniruzzaman 4 , Masahiro Goto 1, 3, 5
Affiliation
|
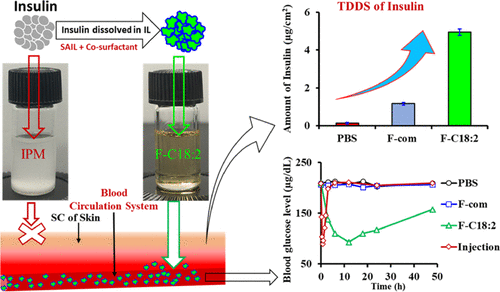
Since injection administration for diabetes is invasive, it is important to develop an effective transdermal method for insulin. However, transdermal delivery remains challenging owing to the strong barrier function of the stratum corneum (SC) of the skin. Here, we developed ionic liquid (IL)-in-oil microemulsion formulations (MEFs) for transdermal insulin delivery using choline–fatty acids ([Chl][FAs])—comprising three different FAs (C18:0, C18:1, and C18:2)—as biocompatible surface-active ILs (SAILs). The MEFs were successfully developed using [Chl][FAs] as surfactants, sorbitan monolaurate (Span-20) as a cosurfactant, choline propionate IL as an internal polar phase, and isopropyl myristate as a continuous oil phase. Ternary phase behavior, dynamic light scattering, and transmission electron microscopy studies revealed that MEFs were thermodynamically stable with nanoparticle size. The MEFs significantly enhanced the transdermal permeation of insulin via the intercellular route by compromising the tight lamellar structure of SC lipids through a fluidity-enhancing mechanism. In vivo transdermal administration of low insulin doses (50 IU/kg) to diabetic mice showed that MEFs reduced blood glucose levels (BGLs) significantly compared with a commercial surfactant-based formulation by increasing the bioavailability of insulin in the systemic circulation and sustained the insulin level for a much longer period (half-life > 24 h) than subcutaneous injection (half-life 1.32 h). When [Chl][C18:2] SAIL-based MEF was transdermally administered, it reduced the BGL by 56% of its initial value. The MEFs were biocompatible and nontoxic (cell viability > 90%). They remained stable at room temperature for 3 months and their biological activity was retained for 4 months at 4 °C. We believe SAIL-based MEFs will alter current approaches to insulin therapy and may be a potential transdermal nanocarrier for protein and peptide delivery.
中文翻译:

使用生物相容性离子液体微乳液治疗糖尿病的胰岛素透皮给药系统
由于糖尿病的注射给药是侵入性的,因此开发一种有效的胰岛素透皮方法非常重要。然而,由于皮肤角质层(SC)的强大屏障功能,透皮给药仍然具有挑战性。在此,我们开发了使用胆碱脂肪酸 ([Chl][FAs]) 进行透皮胰岛素递送的离子液体 (IL) 油包微乳液制剂 (MEF),其中包含三种不同的 FA(C18:0、C18:1 和C18:2)—作为生物相容性表面活性 IL (SAIL)。使用[Chl][FAs]作为表面活性剂、失水山梨糖醇单月桂酸酯(Span-20)作为助表面活性剂、丙酸胆碱IL作为内极性相、肉豆蔻酸异丙酯作为连续油相成功开发了MEF。三元相行为、动态光散射和透射电子显微镜研究表明,MEF 在纳米颗粒尺寸下具有热力学稳定性。 MEF 通过流动性增强机制损害 SC 脂质的紧密层状结构,从而显着增强胰岛素通过细胞间途径的透皮渗透。对糖尿病小鼠进行低剂量(50 IU/kg)胰岛素体内透皮给药表明,与商业表面活性剂制剂相比,MEF 通过增加体循环中胰岛素的生物利用度并维持胰岛素,显着降低血糖水平(BGL)与皮下注射(半衰期 1.32 小时)相比,其水平持续时间更长(半衰期 > 24 小时)。当透皮施用基于 [Chl][C18:2] SAIL 的 MEF 时,它使 BGL 降低了其初始值的 56%。 MEF 具有生物相容性且无毒(细胞活力 > 90%)。它们在室温下可保持稳定 3 个月,其生物活性在 4 °C 下可保持 4 个月。 我们相信基于 SAIL 的 MEF 将改变当前的胰岛素治疗方法,并可能成为用于蛋白质和肽递送的潜在透皮纳米载体。
更新日期:2021-09-15
中文翻译:

使用生物相容性离子液体微乳液治疗糖尿病的胰岛素透皮给药系统
由于糖尿病的注射给药是侵入性的,因此开发一种有效的胰岛素透皮方法非常重要。然而,由于皮肤角质层(SC)的强大屏障功能,透皮给药仍然具有挑战性。在此,我们开发了使用胆碱脂肪酸 ([Chl][FAs]) 进行透皮胰岛素递送的离子液体 (IL) 油包微乳液制剂 (MEF),其中包含三种不同的 FA(C18:0、C18:1 和C18:2)—作为生物相容性表面活性 IL (SAIL)。使用[Chl][FAs]作为表面活性剂、失水山梨糖醇单月桂酸酯(Span-20)作为助表面活性剂、丙酸胆碱IL作为内极性相、肉豆蔻酸异丙酯作为连续油相成功开发了MEF。三元相行为、动态光散射和透射电子显微镜研究表明,MEF 在纳米颗粒尺寸下具有热力学稳定性。 MEF 通过流动性增强机制损害 SC 脂质的紧密层状结构,从而显着增强胰岛素通过细胞间途径的透皮渗透。对糖尿病小鼠进行低剂量(50 IU/kg)胰岛素体内透皮给药表明,与商业表面活性剂制剂相比,MEF 通过增加体循环中胰岛素的生物利用度并维持胰岛素,显着降低血糖水平(BGL)与皮下注射(半衰期 1.32 小时)相比,其水平持续时间更长(半衰期 > 24 小时)。当透皮施用基于 [Chl][C18:2] SAIL 的 MEF 时,它使 BGL 降低了其初始值的 56%。 MEF 具有生物相容性且无毒(细胞活力 > 90%)。它们在室温下可保持稳定 3 个月,其生物活性在 4 °C 下可保持 4 个月。 我们相信基于 SAIL 的 MEF 将改变当前的胰岛素治疗方法,并可能成为用于蛋白质和肽递送的潜在透皮纳米载体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号