当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Organocatalyzed aza-Michael Addition Reaction of 2-Hydroxybenzophenone Imines to Nitroolefins under Batch and Flow Conditions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-06-21 , DOI: 10.1002/adsc.202100635 Andrea Guerrero 1 , Miguel Valle 1 , Alberto Fraile 2 , Jose Julian Aleman Lara 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-06-21 , DOI: 10.1002/adsc.202100635 Andrea Guerrero 1 , Miguel Valle 1 , Alberto Fraile 2 , Jose Julian Aleman Lara 1
Affiliation
|
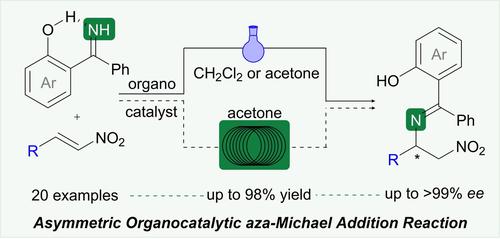
Herein, an asymmetric organocatalytic aza-Michael addition reaction of ketimines to nitroolefins is presented. The use of 2-hydroxybenzophenone imine improves the enantioselective addition of N-centered nucleophiles to nitroalkenes by means of intramolecular hydrogen bond formation at the imine moiety. Moreover, the versatility of the process is demonstrated under both batch and flow conditions, showing the synthesis of a large variety of nitroamine derivatives with excellent yields and enantioselectivities. In addition, we applied this methodology to the formal synthesis of VNI, a drug-like scaffold for the treatment of Chagas disease.
中文翻译:

2-羟基二苯甲酮亚胺与硝基烯烃在间歇和流动条件下的对映选择性有机催化氮杂-迈克尔加成反应
在此,提出了酮亚胺与硝基烯烃的不对称有机催化氮杂-迈克尔加成反应。使用 2-羟基二苯甲酮亚胺通过在亚胺部分形成分子内氢键来改善N中心亲核试剂对硝基烯烃的对映选择性加成。此外,该工艺的多功能性在间歇和流动条件下都得到了证明,显示出多种硝基胺衍生物的合成,具有优异的产率和对映选择性。此外,我们将这种方法应用于 VNI 的正式合成,VNI 是一种用于治疗恰加斯病的药物样支架。
更新日期:2021-08-05
中文翻译:

2-羟基二苯甲酮亚胺与硝基烯烃在间歇和流动条件下的对映选择性有机催化氮杂-迈克尔加成反应
在此,提出了酮亚胺与硝基烯烃的不对称有机催化氮杂-迈克尔加成反应。使用 2-羟基二苯甲酮亚胺通过在亚胺部分形成分子内氢键来改善N中心亲核试剂对硝基烯烃的对映选择性加成。此外,该工艺的多功能性在间歇和流动条件下都得到了证明,显示出多种硝基胺衍生物的合成,具有优异的产率和对映选择性。此外,我们将这种方法应用于 VNI 的正式合成,VNI 是一种用于治疗恰加斯病的药物样支架。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号