当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of Diazomethyl Radicals by Hydrogen Atom Abstraction and Their Cycloaddition with Alkenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-27 , DOI: 10.1002/anie.202105472 Yong-Liang Su 1 , Kuiyong Dong 1 , Haifeng Zheng 1 , Michael P Doyle 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-05-27 , DOI: 10.1002/anie.202105472 Yong-Liang Su 1 , Kuiyong Dong 1 , Haifeng Zheng 1 , Michael P Doyle 1
Affiliation
|
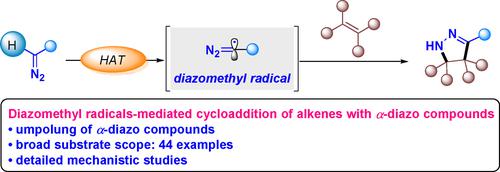
A general catalytic methodology for the synthesis of pyrazolines from α-diazo compounds and conjugated alkenes is reported. The direct hydrogen atom transfer (HAT) process of α-diazo compounds promoted by the tert-butylperoxy radical generates electrophilic diazomethyl radicals, thereby reversing the reactivity of the carbon atom attached with the diazo group. The regiocontrolled addition of diazomethyl radicals to carbon-carbon double bonds followed by intramolecular ring closure on the terminal diazo nitrogen and tautomerization affords a diverse set of pyrazolines in good yields with excellent regioselectivity. This strategy overcomes the limitations of electron-deficient alkenes in traditional dipolar [3+2]-cycloaddition of α-diazo compounds with alkenes. Furthermore, the straightforward formation of the diazomethyl radicals provides umpolung reactivity, thus opening new opportunities for the versatile transformations of diazo compounds.
中文翻译:

氢原子抽提生成重氮甲基自由基及其与烯烃的环加成反应
报道了从 α-重氮化合物和共轭烯烃合成吡唑啉的通用催化方法。叔促进α-重氮化合物的直接氢原子转移(HAT)过程-丁基过氧自由基产生亲电重氮甲基自由基,从而逆转与重氮基团相连的碳原子的反应性。重氮甲基自由基在碳-碳双键上的区域控制加成,然后在末端重氮氮上进行分子内闭环和互变异构化,以良好的收率和出色的区域选择性提供了多种吡唑啉。该策略克服了α-重氮化合物与烯烃的传统偶极[3+2]-环加成中缺电子烯烃的局限性。此外,重氮甲基自由基的直接形成提供了umpolung反应性,从而为重氮化合物的多功能转化开辟了新的机会。
更新日期:2021-05-27
中文翻译:

氢原子抽提生成重氮甲基自由基及其与烯烃的环加成反应
报道了从 α-重氮化合物和共轭烯烃合成吡唑啉的通用催化方法。叔促进α-重氮化合物的直接氢原子转移(HAT)过程-丁基过氧自由基产生亲电重氮甲基自由基,从而逆转与重氮基团相连的碳原子的反应性。重氮甲基自由基在碳-碳双键上的区域控制加成,然后在末端重氮氮上进行分子内闭环和互变异构化,以良好的收率和出色的区域选择性提供了多种吡唑啉。该策略克服了α-重氮化合物与烯烃的传统偶极[3+2]-环加成中缺电子烯烃的局限性。此外,重氮甲基自由基的直接形成提供了umpolung反应性,从而为重氮化合物的多功能转化开辟了新的机会。






























 京公网安备 11010802027423号
京公网安备 11010802027423号