Phytochemistry Letters ( IF 1.3 ) Pub Date : 2021-05-21 , DOI: 10.1016/j.phytol.2021.05.001 Ilkay Erdogan Orhan , Fatma Tosun , Fatma Sezer Senol Deniz , Gokcen Eren , Feyyaz Mıhoğlugil , Demet Akalgan , Mahmut Miski
|
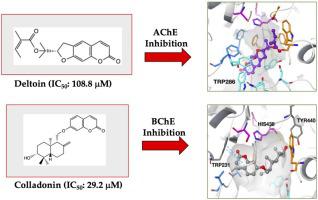
Seventeen natural coumarin derivatives; badrakemin (1), 14′-acetoxybadrakemin (2), badrakemone (3), 14′-acetoxybadrakemone (4), colladonin (5), colladonin acetate (6), 14′-acetoxycolladonin (7), karatavicinol (8), deltoin (9), smyrnioridin (10), marmesin (11), osthol (12), oxypeucedanin (13), oxypeucedanin hydrate (14), isoimperatorin (15), scopoletin (16), and umbelliprenin (17), were tested against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), the sister enzymes that play a critical role in the pathology of Alzheimer’s disease as well as tyrosinase (TYR) as the target for Parkinson’s disease. The tested coumarins were more selective against BChE, where the coumarins 2, 5, 8, and 15 (IC50 = 30.3 μM, 29.2 μM, 37.2 μM, and 50.1 μM, respectively) displayed higher BChE inhibition than the reference (galanthamine, IC50 = 60.2 μM) at 100 μg/mL. Only four coumarins (2, 5, 9, and 15) showed inhibition against AChE. Binding conformations of the coumarins (2, 5, 8, 9, and 15) within the active sites of AChE and BChE were explored via molecular docking experiments. The docked compounds were oriented by the interactions with the oxyanion hole and the peripheral anionic site residues of AChE/BChE. The coumarin derivatives 1–17 was found to have no or low inhibition (2.03 ± 0.92 %–12.91 ± 0.40 %) against TYR at 100 μg/mL. Our findings revealed that coumarins could be promising lead compounds for designing novel anti-Alzheimer drug candidates.
中文翻译:

抑制丁酰胆碱酯酶的天然香豆素分子作为潜在的先导
十七种天然香豆素衍生物;Badrakemin(1),14'- acetoxybadrakemin (2),Badrakemone(3),14'- acetoxybadrakemone(4),colladonin(5),colladoninacetate(6),14'-acetoxycolladonin(7),karatavicinol(8), deltoin(9),smyrnioridin(10),marmesin(11),蛇床子(12),oxypeucedanin(13),oxypeucedanin水合物(14),异欧前胡素(15),东莨菪亭(16),和umbelliprenin(17)对乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)进行了测试,后者在阿尔茨海默氏病的病理学中起着关键作用,而酪氨酸酶(TYR)作为帕金森氏病的靶标。所测试的香豆素是更具选择性的针对的BChE,其中香豆素2,5,8,和15(IC 50 = 30.3μM,29.2μM,37.2微米,50.1微米,分别地)显示更高的BChE抑制比基准(加兰他敏,IC 50 = 60.2μM),浓度为100μg/ mL。只有四个香豆素(2,5,9,和15)显示出对AChE的抑制作用。香豆素(结合构象2,5,8,9,和15的AChE和的BChE的活性位点内)进行了探讨通过分子对接实验。对接的化合物通过与氧阴离子孔和AChE / BChE的周围阴离子位点残基的相互作用而取向。发现100μg/ mL的香豆素衍生物1-17对TYR没有抑制作用或抑制作用很小(2.03±0.92%–12.91±0.40%)。我们的发现表明,香豆素在设计新型抗阿尔茨海默病候选药物方面可能是有前途的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号