当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Evaluation of WD-Repeat-Containing Protein 5 (WDR5) Degraders
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-05-13 , DOI: 10.1021/acs.jmedchem.1c00146 Anja Dölle 1, 2 , Bikash Adhikari 3 , Andreas Krämer 1, 2 , Janik Weckesser 1, 2 , Nicola Berner 4, 5 , Lena-Marie Berger 1, 2 , Mathias Diebold 6 , Magdalena M Szewczyk 7 , Dalia Barsyte-Lovejoy 7, 8 , Cheryl H Arrowsmith 7, 9, 10 , Jakob Gebel 1, 11 , Frank Löhr 1, 11 , Volker Dötsch 1, 10 , Martin Eilers 12 , Stephanie Heinzlmeir 4 , Bernhard Kuster 4, 5, 13 , Christoph Sotriffer 6 , Elmar Wolf 3 , Stefan Knapp 1, 2, 5
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-05-13 , DOI: 10.1021/acs.jmedchem.1c00146 Anja Dölle 1, 2 , Bikash Adhikari 3 , Andreas Krämer 1, 2 , Janik Weckesser 1, 2 , Nicola Berner 4, 5 , Lena-Marie Berger 1, 2 , Mathias Diebold 6 , Magdalena M Szewczyk 7 , Dalia Barsyte-Lovejoy 7, 8 , Cheryl H Arrowsmith 7, 9, 10 , Jakob Gebel 1, 11 , Frank Löhr 1, 11 , Volker Dötsch 1, 10 , Martin Eilers 12 , Stephanie Heinzlmeir 4 , Bernhard Kuster 4, 5, 13 , Christoph Sotriffer 6 , Elmar Wolf 3 , Stefan Knapp 1, 2, 5
Affiliation
|
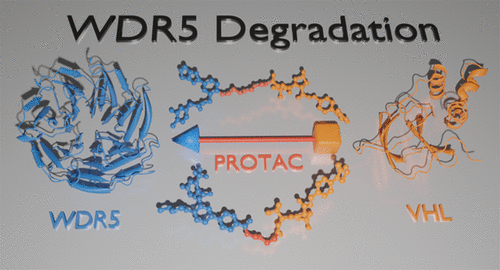
Histone H3K4 methylation serves as a post-translational hallmark of actively transcribed genes and is introduced by histone methyltransferase (HMT) and its regulatory scaffolding proteins. One of these is the WD-repeat-containing protein 5 (WDR5) that has also been associated with controlling long noncoding RNAs and transcription factors including MYC. The wide influence of dysfunctional HMT complexes and the typically upregulated MYC levels in diverse tumor types suggested WDR5 as an attractive drug target. Indeed, protein–protein interface inhibitors for two protein interaction interfaces on WDR5 have been developed. While such compounds only inhibit a subset of WDR5 interactions, chemically induced proteasomal degradation of WDR5 might represent an elegant way to target all oncogenic functions. This study presents the design, synthesis, and evaluation of two diverse WDR5 degrader series based on two WIN site binding scaffolds and shows that linker nature and length strongly influence degradation efficacy.
中文翻译:

含有 WD 重复序列的蛋白质 5 (WDR5) 降解剂的设计、合成和评估
组蛋白 H3K4 甲基化是活跃转录基因的翻译后标志,由组蛋白甲基转移酶 (HMT) 及其调节支架蛋白引入。其中之一是含有 WD 重复序列的蛋白 5 (WDR5),它也与控制长非编码 RNA 和包括 MYC 在内的转录因子有关。功能失调的 HMT 复合物的广泛影响和不同肿瘤类型中通常上调的 MYC 水平表明 WDR5 是一个有吸引力的药物靶点。事实上,已经开发了 WDR5 上两个蛋白质相互作用界面的蛋白质-蛋白质界面抑制剂。虽然此类化合物仅抑制 WDR5 相互作用的一个子集,但 WDR5 的化学诱导蛋白酶体降解可能代表一种针对所有致癌功能的优雅方式。本研究介绍了设计、合成、
更新日期:2021-05-13
中文翻译:

含有 WD 重复序列的蛋白质 5 (WDR5) 降解剂的设计、合成和评估
组蛋白 H3K4 甲基化是活跃转录基因的翻译后标志,由组蛋白甲基转移酶 (HMT) 及其调节支架蛋白引入。其中之一是含有 WD 重复序列的蛋白 5 (WDR5),它也与控制长非编码 RNA 和包括 MYC 在内的转录因子有关。功能失调的 HMT 复合物的广泛影响和不同肿瘤类型中通常上调的 MYC 水平表明 WDR5 是一个有吸引力的药物靶点。事实上,已经开发了 WDR5 上两个蛋白质相互作用界面的蛋白质-蛋白质界面抑制剂。虽然此类化合物仅抑制 WDR5 相互作用的一个子集,但 WDR5 的化学诱导蛋白酶体降解可能代表一种针对所有致癌功能的优雅方式。本研究介绍了设计、合成、





















































 京公网安备 11010802027423号
京公网安备 11010802027423号