当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering Interfacial Chemical and Electrochemical Reactions of Sulfide-Based All-Solid-State Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-05-06 , DOI: 10.1002/aenm.202100210 Changhong Wang 1 , Sooyeon Hwang 2 , Ming Jiang 3 , Jianwen Liang 1 , Yipeng Sun 1 , Keegan Adair 1 , Matthew Zheng 1 , Sankha Mukherjee 3 , Xiaona Li 1 , Ruying Li 1 , Huan Huang 4 , Shangqian Zhao 5 , Li Zhang 5 , Shigang Lu 5 , Jiantao Wang 5 , Chandra Veer Singh 3 , Dong Su 2 , Xueliang Sun 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-05-06 , DOI: 10.1002/aenm.202100210 Changhong Wang 1 , Sooyeon Hwang 2 , Ming Jiang 3 , Jianwen Liang 1 , Yipeng Sun 1 , Keegan Adair 1 , Matthew Zheng 1 , Sankha Mukherjee 3 , Xiaona Li 1 , Ruying Li 1 , Huan Huang 4 , Shangqian Zhao 5 , Li Zhang 5 , Shigang Lu 5 , Jiantao Wang 5 , Chandra Veer Singh 3 , Dong Su 2 , Xueliang Sun 1
Affiliation
|
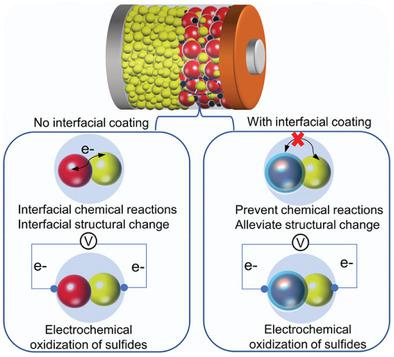
Large interfacial resistance resulting from interfacial reactions is widely acknowledged as one of the main challenges in sulfide electrolytes (SEs)-based all-solid-state lithium batteries (ASSLBs). However, the root cause of the large interfacial resistance between the SEs and typical layered oxide cathodes is not fully understood yet. Here, it is shown that interfacial oxygen loss from single-crystal LiNi0.5Mn0.3Co0.2O2 (SC-NMC532) chemically oxidizes Li10GeP2S12, generating oxygen-containing interfacial species. Meanwhile, the interfacial oxygen loss also induces a structural change of oxide cathodes (layered-to-rock salt). In addition, the high operation voltage can electrochemically oxidize SEs to form non-oxygen species (e.g., polysulfides). These chemically and electrochemically oxidized species, together with the interfacial structural change, are responsible for the large interfacial resistance at the cathode interface. More importantly, the widely adopted interfacial coating strategy is effective in suppressing chemically oxidized oxygen-containing species and mitigating the coincident interfacial structural change but is unable to prevent electrochemically induced non-oxygen species. These findings provide a deeper insight into the large interfacial resistance between the typical SE and layered oxide cathodes, which may be of assistance for the rational interface design of SE-based ASSLBs in the future.
中文翻译:

解读硫化物基全固态电池的界面化学和电化学反应
界面反应导致的大界面电阻被广泛认为是基于硫化物电解质(SE)的全固态锂电池(ASSLB)的主要挑战之一。然而,SEs 和典型的层状氧化物阴极之间的大界面电阻的根本原因尚不完全清楚。在这里,表明来自单晶 LiNi 0.5 Mn 0.3 Co 0.2 O 2 (SC-NMC532) 的界面氧损失化学氧化 Li 10 GeP 2 S 12,产生含氧界面物质。同时,界面氧损失也引起氧化物阴极(层状岩盐)的结构变化。此外,高工作电压可以电化学氧化 SEs 以形成非氧物质(例如,多硫化物)。这些化学和电化学氧化的物质,连同界面结构的变化,是造成阴极界面处大的界面电阻的原因。更重要的是,广泛采用的界面涂层策略可有效抑制化学氧化的含氧物质并减轻同时发生的界面结构变化,但无法防止电化学诱导的非氧物质。
更新日期:2021-06-24
中文翻译:

解读硫化物基全固态电池的界面化学和电化学反应
界面反应导致的大界面电阻被广泛认为是基于硫化物电解质(SE)的全固态锂电池(ASSLB)的主要挑战之一。然而,SEs 和典型的层状氧化物阴极之间的大界面电阻的根本原因尚不完全清楚。在这里,表明来自单晶 LiNi 0.5 Mn 0.3 Co 0.2 O 2 (SC-NMC532) 的界面氧损失化学氧化 Li 10 GeP 2 S 12,产生含氧界面物质。同时,界面氧损失也引起氧化物阴极(层状岩盐)的结构变化。此外,高工作电压可以电化学氧化 SEs 以形成非氧物质(例如,多硫化物)。这些化学和电化学氧化的物质,连同界面结构的变化,是造成阴极界面处大的界面电阻的原因。更重要的是,广泛采用的界面涂层策略可有效抑制化学氧化的含氧物质并减轻同时发生的界面结构变化,但无法防止电化学诱导的非氧物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号