Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MyoD induces ARTD1 and nucleoplasmic poly-ADP-ribosylation during fibroblast to myoblast transdifferentiation
iScience ( IF 4.6 ) Pub Date : 2021-04-17 , DOI: 10.1016/j.isci.2021.102432 Lavinia Bisceglie 1, 2 , Ann-Katrin Hopp 1 , Kapila Gunasekera 1 , Roni H Wright 3, 4 , François Le Dily 3 , Enrique Vidal 3 , Alessandra Dall'Agnese 5 , Luca Caputo 5 , Chiara Nicoletti 5 , Pier Lorenzo Puri 5 , Miguel Beato 3, 6 , Michael O Hottiger 1
iScience ( IF 4.6 ) Pub Date : 2021-04-17 , DOI: 10.1016/j.isci.2021.102432 Lavinia Bisceglie 1, 2 , Ann-Katrin Hopp 1 , Kapila Gunasekera 1 , Roni H Wright 3, 4 , François Le Dily 3 , Enrique Vidal 3 , Alessandra Dall'Agnese 5 , Luca Caputo 5 , Chiara Nicoletti 5 , Pier Lorenzo Puri 5 , Miguel Beato 3, 6 , Michael O Hottiger 1
Affiliation
|
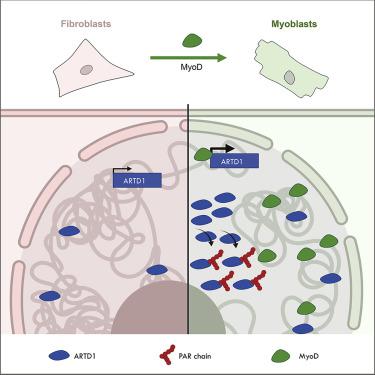
While protein ADP-ribosylation was reported to regulate differentiation and dedifferentiation, it has so far not been studied during transdifferentiation. Here, we found that MyoD-induced transdifferentiation of fibroblasts to myoblasts promotes the expression of the ADP-ribosyltransferase ARTD1 . Comprehensive analysis of the genome architecture by Hi-C and RNA-seq analysis during transdifferentiation indicated that ARTD1 locally contributed to A/B compartmentalization and coregulated a subset of MyoD target genes that were however not sufficient to alter transdifferentiation. Surprisingly, the expression of ARTD1 was accompanied by the continuous synthesis of nuclear ADP ribosylation that was neither dependent on the cell cycle nor induced by DNA damage. Conversely to the H2 O2 -induced ADP-ribosylation, the MyoD-dependent ADP-ribosylation was not associated to chromatin but rather localized to the nucleoplasm. Together, these data describe a MyoD-induced nucleoplasmic ADP-ribosylation that is observed particularly during transdifferentiation and thus potentially expands the plethora of cellular processes associated with ADP-ribosylation.
中文翻译:

MyoD 在成纤维细胞向成肌细胞转分化过程中诱导 ARTD1 和核质聚 ADP 核糖基化
虽然据报道蛋白质 ADP-核糖基化可调节分化和去分化,但迄今为止尚未在转分化过程中对其进行研究。在这里,我们发现MyoD诱导的成纤维细胞向成肌细胞的转分化促进ADP-核糖基转移酶ARTD1的表达。在转分化过程中通过 Hi-C 和 RNA-seq 分析对基因组结构进行综合分析表明,ARTD1 局部促进 A/B 区室化,并共同调节 MyoD 靶基因的子集,但这些基因不足以改变转分化。令人惊讶的是,ARTD1的表达伴随着核ADP核糖基化的持续合成,这种合成既不依赖于细胞周期,也不是由DNA损伤诱导的。与 H2O2 诱导的 ADP 核糖基化相反,MyoD 依赖性 ADP 核糖基化与染色质无关,而是定位于核质。总之,这些数据描述了 MyoD 诱导的核质 ADP-核糖基化,特别是在转分化过程中观察到,因此可能扩展与 ADP-核糖基化相关的大量细胞过程。
更新日期:2021-04-17
中文翻译:

MyoD 在成纤维细胞向成肌细胞转分化过程中诱导 ARTD1 和核质聚 ADP 核糖基化
虽然据报道蛋白质 ADP-核糖基化可调节分化和去分化,但迄今为止尚未在转分化过程中对其进行研究。在这里,我们发现MyoD诱导的成纤维细胞向成肌细胞的转分化促进ADP-核糖基转移酶ARTD1的表达。在转分化过程中通过 Hi-C 和 RNA-seq 分析对基因组结构进行综合分析表明,ARTD1 局部促进 A/B 区室化,并共同调节 MyoD 靶基因的子集,但这些基因不足以改变转分化。令人惊讶的是,ARTD1的表达伴随着核ADP核糖基化的持续合成,这种合成既不依赖于细胞周期,也不是由DNA损伤诱导的。与 H2O2 诱导的 ADP 核糖基化相反,MyoD 依赖性 ADP 核糖基化与染色质无关,而是定位于核质。总之,这些数据描述了 MyoD 诱导的核质 ADP-核糖基化,特别是在转分化过程中观察到,因此可能扩展与 ADP-核糖基化相关的大量细胞过程。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号