当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular Synthesis of Pentagonal and Hexagonal Ring-Fused NBN-Phenalenes Leading to an Excited-State Aromatization-Induced Structural Planarization Molecular Library
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-04-07 , DOI: 10.1021/jacs.1c01339 Cheng-Wei Ju 1 , Bo Li 1 , Lianghui Li 1 , Weiguang Yan 1 , Chunming Cui 1 , Xiaonan Ma 2 , Dongbing Zhao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-04-07 , DOI: 10.1021/jacs.1c01339 Cheng-Wei Ju 1 , Bo Li 1 , Lianghui Li 1 , Weiguang Yan 1 , Chunming Cui 1 , Xiaonan Ma 2 , Dongbing Zhao 1
Affiliation
|
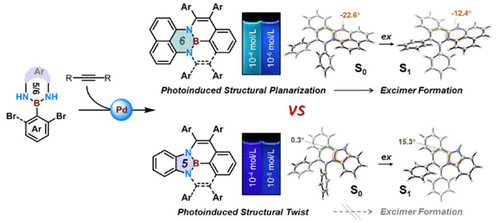
Although polycyclic aromatic hydrocarbons (PAHs) with a nitrogen–boron–nitrogen (NBN) moiety have recently attracted tremendous interest due to their intriguing electronic and optoelectronic properties, all of the NBN-fused π-systems reported to date are called NBN-dibenzophenalenes and were synthesized by electrophilic aromatic substitution. The synthesis of NBN-phenalenes remains challenging, and transition-metal catalysis has never been utilized to construct NBN-embedded π-scaffolds. Herein, a palladium-catalyzed cyclization/bicyclization strategy was developed for the synthesis of diverse pentagonal and hexagonal ring-fused NBN-phenalenes and half-NBN-phenalenes. All of the NBN-embedded π-scaffolds presented in our paper are fluorescent in both solution and the solid state. Further investigations showed that the five-membered NBN rings exhibit the properties of traditional luminogens, while those with a six-membered NBN ring generally undergo photoinduced structural planarization (PISP) and exhibit different colors and quantum yields of fluorescence with different concentrations in solution. Time-resolved spectroscopy and TD-DFT calculations revealed that excited-state aromatization is the driving force for PISP in hexagonal ring-fused NBN-π systems, leading to the formation of excimers. Notably, the scope of PISP compounds is still quite limited, and PISP has never been observed in NBN-π systems before. These hexagonal ring-fused NBN-π systems constitute a novel PISP molecular library and appear to be a new class of aggregation-induced excimer emission (AIEE) materials. Finally, the AIEE behavior of these six-membered NBN rings was applied to the detection of nitro explosives, achieving excellent sensitivity. In general, this work provides a new viewpoint for synthesizing NBN-fused π-systems and understanding the excited-state motion of luminogens.
中文翻译:

五边形和六边形环稠合NBN-苯的模块化合成导致激发态芳构化诱导的结构平面化分子库
尽管具有氮-硼-氮 (NBN) 部分的多环芳烃 (PAH) 由于其有趣的电子和光电特性而最近引起了极大的兴趣,但迄今为止报道的所有 NBN 稠合 π 系统都被称为 NBN-二苯并苯和是通过亲电芳香取代合成的。NBN-phenalenes 的合成仍然具有挑战性,过渡金属催化从未被用于构建 NBN 嵌入的 π-支架。在此,开发了一种钯催化的环化/双环化策略,用于合成各种五边形和六边形环稠合 NBN-phenalenes 和 half-NBN-phenalenes。我们论文中介绍的所有 NBN 嵌入的 π 支架在溶液和固态下都发荧光。进一步的研究表明,五元 NBN 环表现出传统发光体的特性,而六元 NBN 环通常会经历光诱导结构平面化 (PISP),并在溶液中不同浓度时表现出不同颜色和量子产率的荧光。时间分辨光谱和 TD-DFT 计算表明,激发态芳构化是六方环稠合 NBN-π 系统中 PISP 的驱动力,导致准分子的形成。值得注意的是,PISP 化合物的范围仍然非常有限,之前在 NBN-π 系统中从未观察到 PISP。这些六角环稠合 NBN-π 系统构成了一个新的 PISP 分子库,似乎是一类新的聚集诱导准分子发射 (AIEE) 材料。最后,将这些六元 NBN 环的 AIEE 行为应用于硝基爆炸物的检测,实现了出色的灵敏度。总的来说,这项工作为合成 NBN 融合的 π 系统和理解发光体的激发态运动提供了一个新的视角。
更新日期:2021-04-21
中文翻译:

五边形和六边形环稠合NBN-苯的模块化合成导致激发态芳构化诱导的结构平面化分子库
尽管具有氮-硼-氮 (NBN) 部分的多环芳烃 (PAH) 由于其有趣的电子和光电特性而最近引起了极大的兴趣,但迄今为止报道的所有 NBN 稠合 π 系统都被称为 NBN-二苯并苯和是通过亲电芳香取代合成的。NBN-phenalenes 的合成仍然具有挑战性,过渡金属催化从未被用于构建 NBN 嵌入的 π-支架。在此,开发了一种钯催化的环化/双环化策略,用于合成各种五边形和六边形环稠合 NBN-phenalenes 和 half-NBN-phenalenes。我们论文中介绍的所有 NBN 嵌入的 π 支架在溶液和固态下都发荧光。进一步的研究表明,五元 NBN 环表现出传统发光体的特性,而六元 NBN 环通常会经历光诱导结构平面化 (PISP),并在溶液中不同浓度时表现出不同颜色和量子产率的荧光。时间分辨光谱和 TD-DFT 计算表明,激发态芳构化是六方环稠合 NBN-π 系统中 PISP 的驱动力,导致准分子的形成。值得注意的是,PISP 化合物的范围仍然非常有限,之前在 NBN-π 系统中从未观察到 PISP。这些六角环稠合 NBN-π 系统构成了一个新的 PISP 分子库,似乎是一类新的聚集诱导准分子发射 (AIEE) 材料。最后,将这些六元 NBN 环的 AIEE 行为应用于硝基爆炸物的检测,实现了出色的灵敏度。总的来说,这项工作为合成 NBN 融合的 π 系统和理解发光体的激发态运动提供了一个新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号