Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-04-06 , DOI: 10.1016/j.apcatb.2021.120194 Bo Jin , Hilde Poelman , Christophe Detavernier , Zhiwu Liang , Guy B. Marin , Vladimir V. Galvita
|
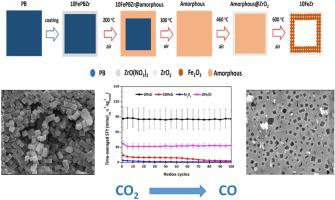
This work provides a way to design oxygen carriers for chemical looping. Starting from Prussian blue microcubes, Zr-promoted Fe-based materials are developed for the conversion of CO2 into CO. Thermally induced oxidative decomposition of ZrO2-coated Prussian blue under the protection of the firstly formed ZrO2 shell leads to the formation of hollow structured materials.
Detailed activity tests and in-situ characterization are used to investigate the relationship between material structure, stability and CO yield from CO2 conversion. Throughout one hundred H2-CO2 redox cycles, 10 %Fe2O3/ZrO2 exhibits a 27 times higher CO space-time yield () than unpromoted, yet hollow structured Fe2O3. This superior performance derives from the dual role that ZrO2 plays: protecting the structure, thereby improving the iron oxide sintering resistance, and facilitating the reduction of iron oxide during redox cycles. The hollow structure and the interaction between Fe2O3 and ZrO2 are the main reasons for the enhanced redox activity.
中文翻译:

氧化铁的微结构ZrO 2涂层可提高CO 2转化率
这项工作为设计用于化学环的氧气载体提供了一种方法。从普鲁士蓝微立方开始,开发了Zr促进的铁基材料,用于将CO 2转化为CO。在首先形成的ZrO 2壳的保护下,热诱导的ZrO 2包覆的普鲁士蓝的氧化分解导致形成Pr 。空心结构材料。
详细的活性测试和原位表征用于研究材料结构,稳定性和由CO 2转化产生的CO产量之间的关系。在整个一百个H 2 -CO 2氧化还原循环中,10%Fe 2 O 3 / ZrO 2的CO时空产率高27倍(),而不是空心的结构化的Fe 2 O 3。ZrO 2起到双重作用,即具有优异的性能:保护结构,从而提高氧化铁的烧结抗性,并促进氧化还原循环中氧化铁的还原。空心结构以及Fe 2 O 3和ZrO 2之间的相互作用是氧化还原活性增强的主要原因。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号