当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissolution–Precipitation Dynamics in Ester Electrolyte for High-Stability Lithium Metal Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-03-23 , DOI: 10.1021/acsenergylett.1c00149 Huicong Yang 1, 2 , Xiang Chen 3 , Nan Yao 3 , Nan Piao 1, 2 , Zhenxing Wang 1, 2 , Kuang He 1, 2 , Hui-Ming Cheng 1, 2, 4 , Feng Li 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-03-23 , DOI: 10.1021/acsenergylett.1c00149 Huicong Yang 1, 2 , Xiang Chen 3 , Nan Yao 3 , Nan Piao 1, 2 , Zhenxing Wang 1, 2 , Kuang He 1, 2 , Hui-Ming Cheng 1, 2, 4 , Feng Li 1, 2
Affiliation
|
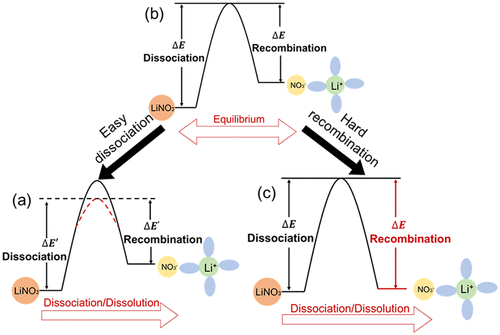
The dissolution of more LiNO3 in an ester electrolyte is a promising way to stabilize the lithium metal anode in a lithium metal battery. Considering that the maximum solubility of LiNO3 is determined by the dynamic equilibrium between the dissociation (dissolution) of LiNO3 and the recombination (precipitation) of solvated Li+ and NO3–, we report a solvation structure that prevents the recombination of anions and cations by ethylene glycol diacetate with low permittivity mixed with ethylene carbonate as cosolvent. The uncoordinated carbonyl of ethylene glycol diacetate in the solvation structure prevents the recombination of cations and anions. The new dissociation–recombination equilibrium establishes, and the solubility of LiNO3 increases. Therefore, the designed ester electrolyte with high LiNO3 solubility achieves lithium metal batteries with high Coulombic efficiency and long cycling life. Our findings show that the solvents with low permittivity can change the solvation structures of cations and increase the solubility of salts as well by preventing the recombination of anions and cations.
中文翻译:

高稳定性锂金属电池酯电解质中的溶解-沉淀动力学
在酯电解质中溶解更多的LiNO 3是稳定锂金属电池中锂金属阳极的一种有前途的方法。考虑到的LiNO的最大溶解度3通过的LiNO的解离(溶解)之间的动态平衡来确定3溶剂化的Li和重组(沉淀)+和NO 3 -,我们报道了一种溶剂化结构,该结构可防止低介电常数的乙二醇二乙酸酯与碳酸亚乙酯混合作为助溶剂来阴离子和阳离子的重组。乙二醇二乙酸酯中未配位的羰基在溶剂化结构中可防止阳离子和阴离子的重组。建立了新的解离-重组平衡,LiNO 3的溶解度增加。因此,设计的具有高LiNO 3溶解度的酯电解质可实现具有高库仑效率和长循环寿命的锂金属电池。我们的发现表明,低介电常数的溶剂可以通过防止阴离子和阳离子的重组来改变阳离子的溶剂化结构,并增加盐的溶解度。
更新日期:2021-04-09
中文翻译:

高稳定性锂金属电池酯电解质中的溶解-沉淀动力学
在酯电解质中溶解更多的LiNO 3是稳定锂金属电池中锂金属阳极的一种有前途的方法。考虑到的LiNO的最大溶解度3通过的LiNO的解离(溶解)之间的动态平衡来确定3溶剂化的Li和重组(沉淀)+和NO 3 -,我们报道了一种溶剂化结构,该结构可防止低介电常数的乙二醇二乙酸酯与碳酸亚乙酯混合作为助溶剂来阴离子和阳离子的重组。乙二醇二乙酸酯中未配位的羰基在溶剂化结构中可防止阳离子和阴离子的重组。建立了新的解离-重组平衡,LiNO 3的溶解度增加。因此,设计的具有高LiNO 3溶解度的酯电解质可实现具有高库仑效率和长循环寿命的锂金属电池。我们的发现表明,低介电常数的溶剂可以通过防止阴离子和阳离子的重组来改变阳离子的溶剂化结构,并增加盐的溶解度。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号