Cell ( IF 45.5 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.cell.2021.02.002 Michelle M McDonald 1 , Weng Hua Khoo 1 , Pei Ying Ng 2 , Ya Xiao 3 , Jad Zamerli 3 , Peter Thatcher 3 , Wunna Kyaw 4 , Karrnan Pathmanandavel 4 , Abigail K Grootveld 4 , Imogen Moran 4 , Danyal Butt 4 , Akira Nguyen 4 , Alexander Corr 1 , Sean Warren 5 , Maté Biro 6 , Natalie C Butterfield 7 , Siobhan E Guilfoyle 7 , Davide Komla-Ebri 7 , Michael R G Dack 7 , Hannah F Dewhurst 7 , John G Logan 7 , Yongxiao Li 8 , Sindhu T Mohanty 1 , Niall Byrne 1 , Rachael L Terry 1 , Marija K Simic 1 , Ryan Chai 3 , Julian M W Quinn 1 , Scott E Youlten 3 , Jessica A Pettitt 3 , David Abi-Hanna 1 , Rohit Jain 9 , Wolfgang Weninger 10 , Mischa Lundberg 11 , Shuting Sun 12 , Frank H Ebetino 12 , Paul Timpson 5 , Woei Ming Lee 8 , Paul A Baldock 1 , Michael J Rogers 1 , Robert Brink 13 , Graham R Williams 7 , J H Duncan Bassett 7 , John P Kemp 14 , Nathan J Pavlos 2 , Peter I Croucher 1 , Tri Giang Phan 13
|
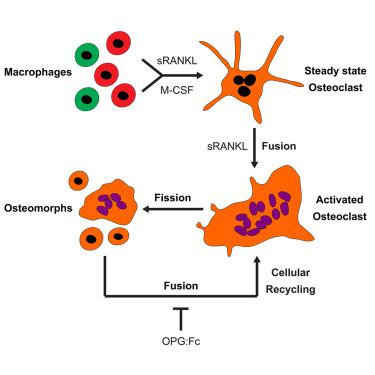
Osteoclasts are large multinucleated bone-resorbing cells formed by the fusion of monocyte/macrophage-derived precursors that are thought to undergo apoptosis once resorption is complete. Here, by intravital imaging, we reveal that RANKL-stimulated osteoclasts have an alternative cell fate in which they fission into daughter cells called osteomorphs. Inhibiting RANKL blocked this cellular recycling and resulted in osteomorph accumulation. Single-cell RNA sequencing showed that osteomorphs are transcriptionally distinct from osteoclasts and macrophages and express a number of non-canonical osteoclast genes that are associated with structural and functional bone phenotypes when deleted in mice. Furthermore, genetic variation in human orthologs of osteomorph genes causes monogenic skeletal disorders and associates with bone mineral density, a polygenetic skeletal trait. Thus, osteoclasts recycle via osteomorphs, a cell type involved in the regulation of bone resorption that may be targeted for the treatment of skeletal diseases.
中文翻译:

在 RANKL 刺激的骨吸收过程中,破骨细胞通过骨形态进行回收
破骨细胞是由单核细胞/巨噬细胞衍生的前体细胞融合形成的大型多核骨吸收细胞,被认为一旦吸收完成就会发生细胞凋亡。在这里,通过活体成像,我们揭示了 RANKL 刺激的破骨细胞具有另一种细胞命运,其中它们分裂成称为骨形态的子细胞。抑制 RANKL 会阻碍这种细胞循环并导致骨形态积累。单细胞RNA测序表明,骨形态在转录上与破骨细胞和巨噬细胞不同,并表达许多非经典破骨细胞基因,这些基因在小鼠体内被删除时与结构和功能性骨表型相关。此外,人类骨形态基因直系同源物的遗传变异会导致单基因骨骼疾病,并与骨矿物质密度(一种多基因骨骼特征)相关。因此,破骨细胞通过骨形态进行回收,骨形态是一种参与骨吸收调节的细胞类型,可能是骨骼疾病治疗的目标。






























 京公网安备 11010802027423号
京公网安备 11010802027423号