当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Hydrogenation of β-Aryl Alkylidene Malonate Esters: Installing an Ester Group Significantly Increases the Efficiency
Organic Letters ( IF 4.9 ) Pub Date : 2021-02-18 , DOI: 10.1021/acs.orglett.1c00093 Qian-Kun Zhao 1 , Xiong Wu 1 , Lin-Ping Li 1 , Fan Yang 1 , Jian-Hua Xie 1 , Qi-Lin Zhou 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-02-18 , DOI: 10.1021/acs.orglett.1c00093 Qian-Kun Zhao 1 , Xiong Wu 1 , Lin-Ping Li 1 , Fan Yang 1 , Jian-Hua Xie 1 , Qi-Lin Zhou 1
Affiliation
|
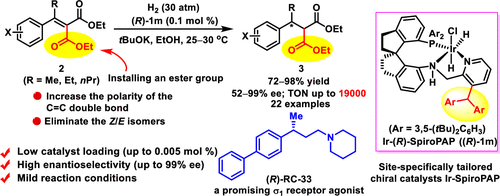
Herein, we report a practical method for efficient asymmetric hydrogenation of β-aryl alkylidene malonates. With a site-specifically tailored chiral spiro iridium catalyst, a series of β-aryl alkylidene malonate esters were hydrogenated to afford chiral malonate esters with good to excellent enantioselectivities (up to 99% ee) and high turnover numbers (up to 19000). The results showed that installing an ester group in α,β-unsaturated carboxylic esters significantly increased the efficiency of their asymmetric hydrogenation reactions.
中文翻译:

β-芳基亚烷基丙二酸酯的不对称加氢:安装酯基显着提高了效率
在此,我们报告了一种有效的不对称氢化β-芳基亚烷基丙二酸酯的实用方法。使用针对特定位置的手性螺铱铱催化剂,一系列β-芳基亚烷基丙二酸酯被氢化,得到手性丙二酸酯,具有良好的对映体选择性(高达99%ee)和高周转率(高达19000)。结果表明,在α,β-不饱和羧酸酯中安装酯基显着提高了其不对称氢化反应的效率。
更新日期:2021-03-05
中文翻译:

β-芳基亚烷基丙二酸酯的不对称加氢:安装酯基显着提高了效率
在此,我们报告了一种有效的不对称氢化β-芳基亚烷基丙二酸酯的实用方法。使用针对特定位置的手性螺铱铱催化剂,一系列β-芳基亚烷基丙二酸酯被氢化,得到手性丙二酸酯,具有良好的对映体选择性(高达99%ee)和高周转率(高达19000)。结果表明,在α,β-不饱和羧酸酯中安装酯基显着提高了其不对称氢化反应的效率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号