当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium(II)-Catalyzed Substituted Pyridine Synthesis from α,β-Unsaturated Oxime Ethers via a C–H Alkenylation/Aza-6π-Electrocyclization Approach
Organic Letters ( IF 4.9 ) Pub Date : 2021-02-10 , DOI: 10.1021/acs.orglett.1c00061 Takahiro Yamada 1 , Yoshimitsu Hashimoto 1 , Kosaku Tanaka 1 , Nobuyoshi Morita 1 , Osamu Tamura 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-02-10 , DOI: 10.1021/acs.orglett.1c00061 Takahiro Yamada 1 , Yoshimitsu Hashimoto 1 , Kosaku Tanaka 1 , Nobuyoshi Morita 1 , Osamu Tamura 1
Affiliation
|
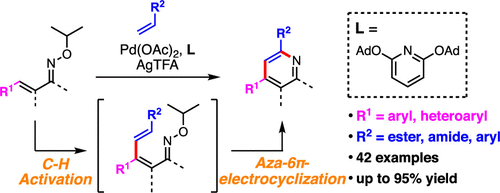
An efficient synthetic method for multisubstituted pyridines from β-aryl-substituted α,β-unsaturated oxime ethers and alkenes via Pd-catalyzed C–H activation has been developed. Systematic optimization of catalyst ligands revealed that sterically hindered pyridines increased the reactivity. Mechanistic studies suggested that the products are formed by Pd-catalyzed β-alkenylation of α,β-unsaturated oxime followed by aza-6π-electrocyclization. Various oximes and alkenes could be utilized to afford multisubstituted pyridines with complete regioselectivity. The usefulness of this methodology was showcased by the synthesis of 4-aryl-substituted pyridine derivatives, which are difficult to access with previously reported Rh-catalyzed approaches with alkenes.
中文翻译:

α–β-不饱和肟醚通过C–H烯基化/Aza-6π-电环化方法催化钯(II)取代的吡啶合成
已经开发了一种有效的合成方法,可通过Pd催化的CH活化从β-芳基取代的α,β-不饱和肟醚和烯烃中合成多取代的吡啶。催化剂配体的系统优化表明,位阻吡啶可提高反应活性。机理研究表明,产物是由Pd催化α,β-不饱和肟的β-烯基化,然后进行aza-6π-电环化而形成的。可以使用各种肟和烯烃来提供具有完全区域选择性的多取代吡啶。该方法的有用性通过4-芳基取代的吡啶衍生物的合成得到了证明,这是以前报道的用烯烃进行的Rh催化方法难以获得的。
更新日期:2021-03-05
中文翻译:

α–β-不饱和肟醚通过C–H烯基化/Aza-6π-电环化方法催化钯(II)取代的吡啶合成
已经开发了一种有效的合成方法,可通过Pd催化的CH活化从β-芳基取代的α,β-不饱和肟醚和烯烃中合成多取代的吡啶。催化剂配体的系统优化表明,位阻吡啶可提高反应活性。机理研究表明,产物是由Pd催化α,β-不饱和肟的β-烯基化,然后进行aza-6π-电环化而形成的。可以使用各种肟和烯烃来提供具有完全区域选择性的多取代吡啶。该方法的有用性通过4-芳基取代的吡啶衍生物的合成得到了证明,这是以前报道的用烯烃进行的Rh催化方法难以获得的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号