Journal of Power Sources ( IF 8.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jpowsour.2021.229504 Dong Woo Kang , Janghyuk Moon , Hae-Young Choi , Heon-Cheol Shin , Byung Gon Kim
|
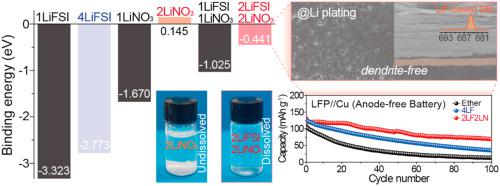
Despite their potential for achieving high energy density, anode-free batteries (AFBs) suffer from poor cycling stability caused by uncontrollable Li-metal deposition. To improve the reversibility of Li by physically preventing Li dendrite growth and minimizing the side reaction area between Li and the free solvent, two representative salts, lithium bis(fluorosulfonyl)imide (LiFSI) forming a robust/conductive LiF-based solid electrolyte interphase (SEI) layer, and LiNO3 altering the deposited Li morphology from dendritic to spherical, are widely used as salts for electrolyte. However, the depletion of LiNO3 by continuous consumption during cycling is a problem due to its low solubility. Thus, here, we report the use of high-concentration dual-salt electrolyte consisting of LiFSI and LiNO3 for AFBs to circumvent these issues. Experimental and theoretical studies reveal that electrolytes with high-concentration LiNO3 (up to 2 M) can be prepared by suppressing the agglomeration of LiNO3 in 1,2-dimethoxyethane (DME) in the presence of LiFSI. Because of the densely packed Li without dendrite, the formation of a protective SEI layer, and the alleviation of electrolyte oxidation as well as Al corrosion, the AFBs with this electrolyte achieve good cycling performance by reversible operation of Li. This work demonstrates the importance of electrolyte engineering for highly stable AFBs.
中文翻译:

稳定的循环和均匀的锂析出游离阳极金属锂电池能够通过具有高的LiNO的高浓度双盐电解质3含量
尽管无阳极电池(AFB)具有实现高能量密度的潜力,但它们由于无法控制的锂金属沉积而遭受循环稳定性差的困扰。为了通过物理上防止Li树枝状晶体生长并最小化Li与游离溶剂之间的副反应区域来提高Li的可逆性,两种代表性的盐双(氟磺酰基)酰亚胺锂(LiFSI)形成了坚固/导电的基于LiF的固体电解质中间相( SEI)层和将沉积的Li形态从树状变为球形的LiNO 3被广泛用作电解质的盐。然而,LiNO 3的耗尽由于其溶解度低,在循环过程中连续消耗而成为一个问题。因此,在这里,我们报道了AFBs使用由LiFSI和LiNO 3组成的高浓度双盐电解质来解决这些问题的方法。实验和理论研究表明,可以通过抑制LiNO 3的团聚来制备高浓度LiNO 3(最高2 M)的电解质在LiFSI存在下,在1,2-二甲氧基乙烷(DME)中溶解。由于锂的密集堆积而没有树枝状结晶,形成了保护性SEI层,并且减轻了电解液的氧化以及铝的腐蚀,因此带有这种电解液的AFB通过可逆的锂操作实现了良好的循环性能。这项工作证明了电解质工程对于高度稳定的AFB的重要性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号