Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2021-01-27 , DOI: 10.1016/j.snb.2021.129563 Zhenzhen Han , Cheng Peng , Jia Yi , Dongxue Zhang , Xiaowei Xiang , Xinyan Peng , Bin Su , Baohong Liu , Yuhui Shen , Liang Qiao
|
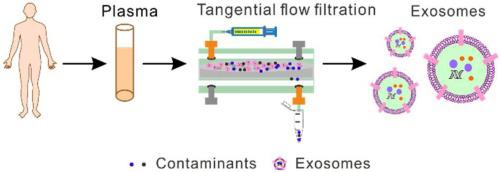
Exosomes play an important role in microenvironmental regulation, cellular communication, tumor progression/metastasis and prognosis. A key challenge in the study of exosomes is the isolation and purification of exosomes from contaminants. In this study, we develop a size-dependent microfluidic chip to isolate and purify exosomes from human blood by tangential flow filtration. The microfluidic chip combines symmetrically two polymethyl methacrylate (PMMA) layers with serpentine channels and a nanoporous polycarbonate track etched (PCTE) membrane in between. Due to the uniform pore size (∼100 nm in diameter) of the nanoporous PCTE membrane, protein contaminants can be cleaned with high efficiency (> 97 %), and exosomes trapped in the microfluidic chip can be collected by reverse elution with high recovery (> 80 %). The collected exosomes are subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) analysis. Based on the fingerprint peaks of exosomes, it is demonstrated that plasma proteins are largely cleaned and only exosome proteins dominate the mass spectra. Compared to ultracentrifugation (UC) that is considered as the “gold standard” for exosome isolation and purification, the size-dependent microfluidic chip-based protocol shows higher protein cleaning efficiency and exosome recovery rate. The whole process takes <3 h, shorter than UC. Considering that microfluidic based methods can be automatically controlled, the current method is promising in large-cohort clinical usage.
中文翻译:

通过基于切向流过滤的微流控芯片从人血浆中高效纯化外泌体
外泌体在微环境调节,细胞通讯,肿瘤进展/转移和预后中起重要作用。外泌体研究中的关键挑战是从污染物中分离和纯化外泌体。在这项研究中,我们开发了一种大小依赖的微流控芯片,通过切向流过滤从人血中分离和纯化外泌体。微流体芯片将两个聚甲基丙烯酸甲酯(PMMA)层对称地结合在一起,并具有蛇形通道和介于两者之间的纳米多孔聚碳酸酯轨迹蚀刻(PCTE)膜。由于纳米多孔PCTE膜的孔径均匀(直径约100 nm),因此可以高效(> 97%)清洗蛋白质污染物,并且可以通过反向洗脱以高回收率收集微流体芯片中捕获的外泌体( > 80%)。对收集的外泌体进行基质辅助激光解吸/电离飞行时间(MALDI-TOF)质谱(MS)分析。基于外泌体的指纹峰,表明血浆蛋白被大量清洗,只有外泌体蛋白在质谱中占主导地位。与被认为是外泌体分离和纯化的“金标准”的超速离心(UC)相比,基于尺寸的微流控芯片方案显示出更高的蛋白质清洁效率和外泌体回收率。整个过程耗时少于3小时,比UC短。考虑到基于微流体的方法可以自动控制,目前的方法在大队列临床应用中很有希望。基于外泌体的指纹峰,表明血浆蛋白被大量清洗,只有外泌体蛋白在质谱中占主导地位。与被认为是外泌体分离和纯化的“金标准”的超速离心(UC)相比,基于尺寸的微流控芯片方案显示出更高的蛋白质清洁效率和外泌体回收率。整个过程耗时少于3小时,比UC短。考虑到基于微流体的方法可以自动控制,目前的方法在大队列临床应用中很有希望。基于外泌体的指纹峰,表明血浆蛋白被大量清洗,只有外泌体蛋白在质谱中占主导地位。与被认为是外泌体分离和纯化的“金标准”的超速离心(UC)相比,基于尺寸的微流控芯片方案显示出更高的蛋白质清洁效率和外泌体回收率。整个过程耗时少于3小时,比UC短。考虑到基于微流体的方法可以自动控制,目前的方法在大队列临床应用中很有希望。基于大小的微流控芯片方案显示出更高的蛋白质清洁效率和外泌体回收率。整个过程耗时少于3小时,比UC短。考虑到基于微流体的方法可以自动控制,目前的方法在大队列临床应用中很有希望。基于大小的微流控芯片方案显示出更高的蛋白质清洁效率和外泌体回收率。整个过程耗时少于3小时,比UC短。考虑到基于微流体的方法可以自动控制,目前的方法在大队列临床应用中很有希望。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号