Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protease Biosensor by Conversion of a Homogeneous Assay into a Surface-Tethered Electrochemical Analysis Based on Streptavidin–Biotin Interactions
ACS Sensors ( IF 8.2 ) Pub Date : 2021-01-22 , DOI: 10.1021/acssensors.0c02415 Ning Xia 1 , Zhifang Sun 1 , Fangyuan Ding 1 , Yanan Wang 1 , Wenna Sun 1 , Lin Liu 1
ACS Sensors ( IF 8.2 ) Pub Date : 2021-01-22 , DOI: 10.1021/acssensors.0c02415 Ning Xia 1 , Zhifang Sun 1 , Fangyuan Ding 1 , Yanan Wang 1 , Wenna Sun 1 , Lin Liu 1
Affiliation
|
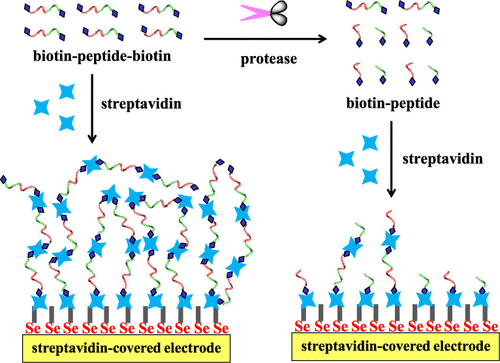
This work proposed a new sensing strategy for protease detection by converting a homogeneous assay into a surface-tethered electrochemical analysis. Streptavidin (SA), a tetramer protein, was used as the sensing unit based on the SA–biotin coupling chemistry. Caspase-3 was used as the model analyte, and a biotinylated peptide with a sequence of biotin–GDEVDGK–biotin was designed as the substrate. Specifically, the peptide substrate could induce an assembly of SA to form (SA–biotin–GDEVDGK–biotin)n aggregates through SA–biotin interactions, which was confirmed by atomic force microscopy (AFM). The peptide substrate-induced assembly of SA was facilely initiated on an electrode–liquid surface by modification of the electrode with SA. The in situ formation of (SA–biotin–GDEVDGK–biotin)n aggregates created an insulating layer, thus limiting the electron transfer of ferricyanide. Once the peptide substrate was cleaved into two shorter fragments (biotin–GDEVD and GK–biotin) by caspase-3, the resulting products would compete with biotin–GDEVDGK–biotin to bind SA proteins immobilized on the electrode surface and distributed in a solution, thus preventing the in situ formation of (SA–biotin–GDEVDGK–biotin)n assemblies. With the simple principle of the substrate-induced assembly of SA, a dual-signal amplification was achieved with improved sensitivity. Taking advantage of high sensitivity, simple principle, and easy operation, this method can be augmented to design various surface-tethered biosensors for practical applications.
中文翻译:

蛋白酶生物传感器通过将均相分析转化为基于链霉亲和素-生物素相互作用的表面束缚电化学分析
这项工作提出了一种新的蛋白酶检测传感策略,可以将同质分析转换为表面束缚的电化学分析。链霉亲和素(SA)是一种四聚体蛋白,基于SA-生物素偶联化学,被用作传感单位。Caspase-3被用作模型分析物,带有生物素–GDEVDGK–生物素序列的生物素化肽被设计为底物。具体而言,肽底物可通过SA-生物素相互作用诱导SA组装,形成SA(生物素-GDEVDGK-生物素)n聚集体,这已通过原子力显微镜(AFM)确认。肽底物诱导的SA组装是通过用SA修饰电极在电极-液体表面上容易地引发的。(SA–生物素–GDEVDGK–生物素)n的原位形成聚集体形成绝缘层,从而限制了铁氰化物的电子转移。一旦肽底物被caspase-3裂解为两个较短的片段(生物素– GDEVD和GK –生物素),所得产物将与生物素– GDEVDGK –生物素竞争结合固定在电极表面并分布在溶液中的SA蛋白,因此可以防止原位形成(SA-生物素-GDEVDGK-生物素)n装配体。利用SA的底物诱导组装的简单原理,可以提高灵敏度,实现双信号放大。利用高灵敏度,简单原理和易于操作的优势,可以扩展该方法,以设计各种用于实际应用的表面束缚式生物传感器。
更新日期:2021-03-26
中文翻译:

蛋白酶生物传感器通过将均相分析转化为基于链霉亲和素-生物素相互作用的表面束缚电化学分析
这项工作提出了一种新的蛋白酶检测传感策略,可以将同质分析转换为表面束缚的电化学分析。链霉亲和素(SA)是一种四聚体蛋白,基于SA-生物素偶联化学,被用作传感单位。Caspase-3被用作模型分析物,带有生物素–GDEVDGK–生物素序列的生物素化肽被设计为底物。具体而言,肽底物可通过SA-生物素相互作用诱导SA组装,形成SA(生物素-GDEVDGK-生物素)n聚集体,这已通过原子力显微镜(AFM)确认。肽底物诱导的SA组装是通过用SA修饰电极在电极-液体表面上容易地引发的。(SA–生物素–GDEVDGK–生物素)n的原位形成聚集体形成绝缘层,从而限制了铁氰化物的电子转移。一旦肽底物被caspase-3裂解为两个较短的片段(生物素– GDEVD和GK –生物素),所得产物将与生物素– GDEVDGK –生物素竞争结合固定在电极表面并分布在溶液中的SA蛋白,因此可以防止原位形成(SA-生物素-GDEVDGK-生物素)n装配体。利用SA的底物诱导组装的简单原理,可以提高灵敏度,实现双信号放大。利用高灵敏度,简单原理和易于操作的优势,可以扩展该方法,以设计各种用于实际应用的表面束缚式生物传感器。





























 京公网安备 11010802027423号
京公网安备 11010802027423号